The philosophers' elements
The four elements of Western alchemy
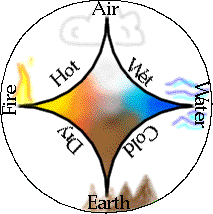
The figure shows how the four elements were imagined to combine in various pairs to produce the "qualities" of hot, cold, wetness and dryness.
To these, Vedic (Hindu) philosophers of India added space, while the ancient Chinese concept of Wu Xing regarded earth, metal, wood, fire and water as fundamental. These basic elements were not generally considered to exist as the actual materials we know as earth, water, etc., but rather to represent the "principles" or essences that the elements conveyed to the various kinds of matter we encounter in the world.
The chemists' elements
Eventually, practical experience (largely connected with the extraction of metals from ores) and the beginnings of scientific experimentation in the 18th Century led to our modern concept of the chemical element.
An element is a substance: the simplest form to which any other chemical substance can be reduced through appropriate thermal or chemical treatment.
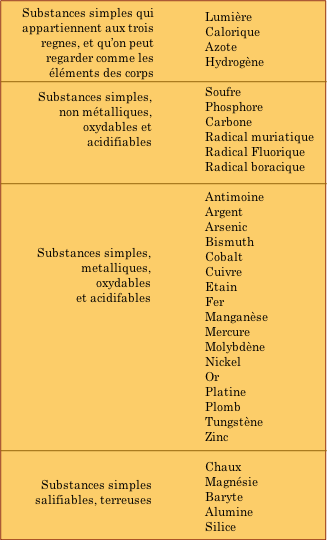 "Simplest", in the context of experimentation at the time, was defined in terms of weight; cinnabar
(mercuric sulfide) can be broken down into two substances, mercury and
sulfur, which themselves cannot be reduced to any lighter forms. The
first textbook of Chemistry, Traitè Èlèmentaire de Chemie, published by Antoine Lavoisier ("the father of Chemistry") in 1789, contained the table of elements shown here.
"Simplest", in the context of experimentation at the time, was defined in terms of weight; cinnabar
(mercuric sulfide) can be broken down into two substances, mercury and
sulfur, which themselves cannot be reduced to any lighter forms. The
first textbook of Chemistry, Traitè Èlèmentaire de Chemie, published by Antoine Lavoisier ("the father of Chemistry") in 1789, contained the table of elements shown here.
Antoine Lavoisier (1743-1794)
Although old Antoine got many of these right, he did
manage to include a few things that don't quite fit into our modern idea
of what constitutes a chemical element. There are two such mistakes in
the top section of the table that you should be able to identify even if
your French is less than tip-top— can you find them?
Lav's other mis-assignment of the elements in the
bottom section was not really his fault. Chalk, magnesia, barytes,
alumina and silica are highly stable oxygen-containing compounds; the
high temperatures required to break them down could not be achieved in
Lavoisier's time. (Magnesia,
after all, is what fire brick is made of!) The proper classification of
these substances was delayed until further experimentation revealed
their true nature.
Ten of the chemical elements have been known since ancient times. Five more were discovered through the 17th Century. Some frequently-asked questions about elements
How many elements are there?
Ninety-two elements have been found in nature. Around 25 more have been made artificially, but all of these decay into lighter elements, with some of them disappearing in minutes or even seconds.Where do the elements come from?
The processes by which elements (or more properly, their nuclei) are formed is known as nucleosynthesis. The very first (primordial) nuclei formed shortly after the "big bang".The present belief is that helium and a few other very light elements were formed within about three minutes of the "big bang", and that the next 23 elements (up through iron) are formed mostly by nuclear fusion processes within stars, in which lighter nuclei combine into successively heavier elements. Elements heavier than iron cannot be formed in this way, and are produced only during the catastrophic collapse of massive stars (supernovae explosions).
How do the elements vary in abundance?
Quite markedly, and very differently in different bodies in the cosmos. Most of the atoms in the universe still consist of hydrogen, with helium being a distant second. On Earth, oxygen, silicon, and aluminum are most abundant. These profiles serve as useful guides for constructing models for the formation of the earth and other planetary bodies. |
Elemental abundances in the lithosphere (Earth's crust) and in the universe.
Note that the vertical axis is logarithmic, which
has the effect of greatly reducing the visual impression of the
differences between the various elements.
|
How did the elements get their names?
This is too big a subject to cover here in detail, especially since most elements have different names in different languages. Here are some useful links:- ← By far the most interesting resource is the Elementymology Multidictionary which covers the history, discovery and naming of each element and a list of the names of each element in 97 languages.
- Wikipedia has a comprehensive list of English element name etymologies and also a Timeline of chemical element discoveries.
- The naming of some of the more recently-discovered (artificially-created) elements has been a matter of some controversy.
- An interesting description of how elements are named in Chinese
How did the element symbols originate?
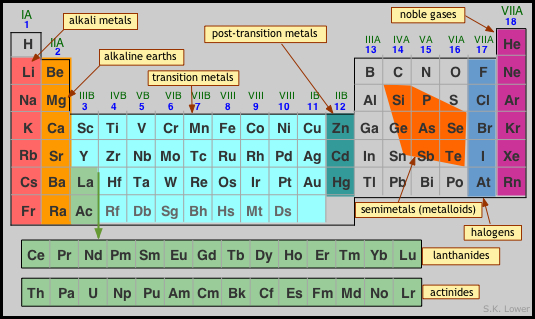
The system of element symbols we use today was established by the Swedish chemist Jöns Jacob Berzelius in 1814. Prior to that time, graphic alchemical symbols were used, which were later modified and popularized by John Dalton (See here). Fortunately for English speakers, the symbols of most of the elements serve as mnemonics for their names, but this is not true for the seven metals known from antiquity, whose symbols are derived from their Latin names. The other exception is tungsten (a name derived from Swedish), whose symbol W reflects the German name which is more widely used.How are the elements organized?
Two general organizing principles developed in the 19th
Century: one was based on the increasing relative weights (atomic
weights) of the elements, yielding a list that begins this way:
H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca...
The other principle took note of the similarities of the
properties of the elements, organizing them into groups with similar
properties (Döbereiner's "triads", 1829). It was later noted that groups of elements (Chancourtois' "telluric helix", Newlands "octaves", 1864 and Meyer,
1869) having changing properties tended to repeat themselves within the
atomic weight sequence, giving rise to the idea of "periodic" sequences
of properties. These concepts were finally integrated into the periodic table published by Mendeleev in 1869, which evolved into the various forms of the periodic table in use today.

No comments:
Post a Comment