Bromine reacts with 2-butene to form 2,3-dibromobutane.
It also reacts with 3-methyl-2-pentene to form 2,3-dibromopentane.
Instead of trying to memorize both equations, we can build a general rule that bromine
reacts with compounds that contain a C=C double bond to give the product expected from
addition across the double bond. This approach to understanding the chemistry of organic
compounds presumes that certain atoms or groups of atoms known as
functional
groups give these compounds their characteristic properties.
Functional groups focus attention on the important aspects of the structure of a
molecule. We don't have to worry about the differences between the structures of 1-butene
and 2-methyl-2-hexene, for example, when these compounds react with hydrogen bromide. We
can focus on the fact that both compounds are alkenes that add HBr across the C=C double
bond in the direction predicted by
Markovnikov's
rule.
Some common functional groups are given in the table below.
Common Functional Groups
| Functional Group |
|
Name |
|
Example |
 |
|
Alkane |
|
CH3CH2CH3 (propane) |
 |
|
Alkene |
|
CH3CH=CH2 (propene) |
 |
|
Alkyne |
|
CH3C CH (propyne) CH (propyne) |
| F, Cl, Br, or I |
|
Alkyl halide |
|
CH3Br (methyl bromide) |
 |
|
Alcohol |
|
CH3CH2OH (ethanol) |
 |
|
Ether |
|
CH3OCH3 (dimethyl ether) |
 |
|
Amine |
|
CH3NH2 (methyl amine) |
The C=O group plays a particularly important role in organic chemistry. This group is
called a
carbonyl and some of the functional groups based on a carbonyl
are shown in the table below.
Functional Groups That Contain a Carbonyl
| Functional Group |
|
Name |
|
Example |
 |
|
Aldehyde |
|
CH3CHO (acetaldehyde) |
 |
|
Ketone |
|
CH3COCH3 (acetone) |
 |
|
Acyl chloride |
|
CH3COCl (acetyl chloride) |
 |
|
Carboxylic acid |
|
CH3CO2H (acetic acid) |
 |
|
Ester |
|
CH3CO2CH3 (methyl acetate) |
 |
|
Amide |
|
CH3NH2 (acetamide) |
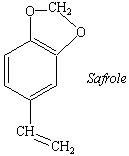
Practice Problem 1:Root beer hasn't tasted the same since
the use of sassafras oil as a food additive was outlawed because sassafras oil is 80%
safrole, which has been shown to cause cancer in rats and mice. Identify the functional
groups in the structure of safrole.
|
Practice Problem 2:The following compounds are the active
ingredients in over-the-counter drugs used as analgesics (to relieve pain without
decreasing sensibility or consciousness), antipyretics (to reduce the body temperature
when it is elevated), and/or anti-inflammatory agents (to counteract swelling or
inflammation of the joints, skin, and eyes). Identify the functional groups in each
molecule.
|
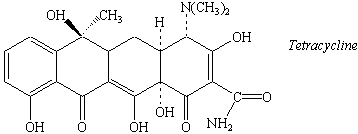
Practice Problem 3:The discovery of penicillin in 1928
marked the beginning of what has been called the "golden age of chemotherapy,"
in which previously life-threatening bacterial infections were transformed into little
more than a source of discomfort. For those who are allergic to penicillin, a variety of
antibiotics, including tetracycline, are available. Identify the numerous functional
groups in the tetracycline molecule.
|
Focusing on the functional groups in a molecule allows us to recognize patterns in the
behavior of related compounds. Consider what we know about the reaction between sodium
metal and water, for example.
2 Na(
s) + 2 H
2O(
l)
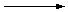
H
2(
g)
+ 2 Na
+(
aq) + 2 OH
-(
aq)
We can divide this reaction into two half-reactions. One involves the oxidation of
sodium metal to form sodium ions.
| Oxidation: |
|
Na |
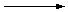 |
Na+ + e- |
The other involves the reduction of an H
+ ion in water to form a neutral
hydrogen atom that combines with another hydrogen atom to form an H
2 molecule.
| Reduction: |
|
 |
Once we recognize that water contains an

OH functional group, we can predict what might happen when sodium metal reacts
with an alcohol that contains the same functional group. Sodium metal should react with
methanol (CH
3OH), for example, to give H
2 gas and a solution of the
Na
+ and CH
3O
- ions dissolved in this alcohol.
2 Na(
s) + 2 CH
3OH(
l)
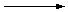
H
2(
g)
+ 2 Na
+(
alc) + 2 CH
3O
-(
alc)
Because they involve the transfer of electrons, the reaction between sodium metal and
either water or an alcohol are examples of oxidation-reduction reactions. But what about
the following reaction, in which hydrogen gas reacts with an alkene in the presence of a
transition metal catalyst to form an alkane?
There is no change in the number of valence electrons on any of the atoms in this
reaction. Both before and after the reaction, each carbon atom shares a total of eight
valence electrons and each hydrogen atom shares two electrons. Instead of electrons, this
reaction involves the transfer of atoms

in this case, hydrogen atoms. There are so many atom-transfer reactions that
chemists developed the concept of
oxidation number to extend the idea of
oxidation and reduction to reactions in which electrons aren't necessarily gained or lost.
| Oxidation involves an increase in the oxidation number of an atom. |
| Reduction occurs when the oxidation number of an atom decreases. |
During the transformation of ethene into ethane, there is a
decrease in the
oxidation number of the carbon atom. This reaction therefore involves the
reduction
of ethene to ethane.
Reactions in which none of the atoms undergo a change in oxidation number are called
metathesis
reactions. Consider the reaction between a carboxylic acid and an amine, for
example.
Or the reaction between an alcohol and hydrogen bromide.
These are metathesis reactions because there is no change in the oxidation
number of any atom in either reaction.
The oxidation numbers of the carbon atoms in a variety of compounds are given in the
table below
.
Typical Oxidation Numbers of Carbon
| Functional Group |
|
Example |
|
Oxidation Number of
Carbon in the Example |
| Alkane |
|
CH4 |
|
-4 |
| Alkyllithium |
|
CH3Li |
|
-4 |
| Alkene |
|
H2C=CH2 |
|
-2 |
| Alcohol |
|
CH3OH |
|
-2 |
| Ether |
|
CH3OCH3 |
|
-2 |
| Alkyl halide |
|
CH3Cl |
|
-2 |
| Amine |
|
CH3NH2 |
|
-2 |
| Alkyne |
|
HC CH CH |
|
-1 |
| Aldehyde |
|
H2CO |
|
0 |
| Carboxylic acid |
|
HCO2H |
|
2 |
|
|
CO2 |
|
4 |
These oxidation numbers can be used to classify organic reactions
as either oxidation-reduction reactions or metathesis reactions.
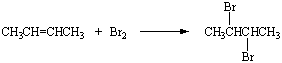 It also reacts with 3-methyl-2-pentene to form 2,3-dibromopentane.
It also reacts with 3-methyl-2-pentene to form 2,3-dibromopentane. 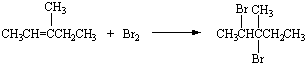 Instead of trying to memorize both equations, we can build a general rule that bromine
reacts with compounds that contain a C=C double bond to give the product expected from
addition across the double bond. This approach to understanding the chemistry of organic
compounds presumes that certain atoms or groups of atoms known as functional
groups give these compounds their characteristic properties.
Instead of trying to memorize both equations, we can build a general rule that bromine
reacts with compounds that contain a C=C double bond to give the product expected from
addition across the double bond. This approach to understanding the chemistry of organic
compounds presumes that certain atoms or groups of atoms known as functional
groups give these compounds their characteristic properties. 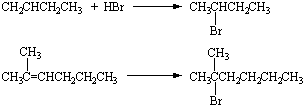
 OH functional group, we can predict what might happen when sodium metal reacts
with an alcohol that contains the same functional group. Sodium metal should react with
methanol (CH3OH), for example, to give H2 gas and a solution of the
Na+ and CH3O- ions dissolved in this alcohol.
OH functional group, we can predict what might happen when sodium metal reacts
with an alcohol that contains the same functional group. Sodium metal should react with
methanol (CH3OH), for example, to give H2 gas and a solution of the
Na+ and CH3O- ions dissolved in this alcohol. 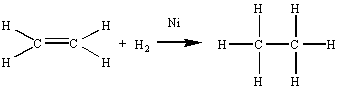 There is no change in the number of valence electrons on any of the atoms in this
reaction. Both before and after the reaction, each carbon atom shares a total of eight
valence electrons and each hydrogen atom shares two electrons. Instead of electrons, this
reaction involves the transfer of atoms
There is no change in the number of valence electrons on any of the atoms in this
reaction. Both before and after the reaction, each carbon atom shares a total of eight
valence electrons and each hydrogen atom shares two electrons. Instead of electrons, this
reaction involves the transfer of atoms in this case, hydrogen atoms. There are so many atom-transfer reactions that
chemists developed the concept of oxidation number to extend the idea of
oxidation and reduction to reactions in which electrons aren't necessarily gained or lost.
in this case, hydrogen atoms. There are so many atom-transfer reactions that
chemists developed the concept of oxidation number to extend the idea of
oxidation and reduction to reactions in which electrons aren't necessarily gained or lost.
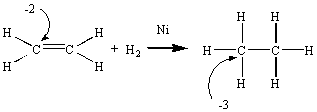 Reactions in which none of the atoms undergo a change in oxidation number are called metathesis
reactions. Consider the reaction between a carboxylic acid and an amine, for
example.
Reactions in which none of the atoms undergo a change in oxidation number are called metathesis
reactions. Consider the reaction between a carboxylic acid and an amine, for
example. 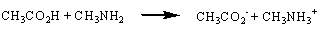
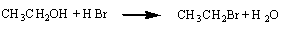
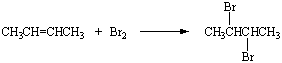
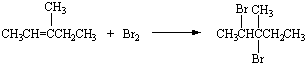
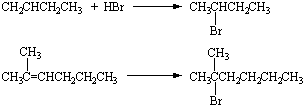


 H2(g)
+ 2 Na+(aq) + 2 OH-(aq)
H2(g)
+ 2 Na+(aq) + 2 OH-(aq) 
 H2(g)
+ 2 Na+(alc) + 2 CH3O-(alc)
H2(g)
+ 2 Na+(alc) + 2 CH3O-(alc) 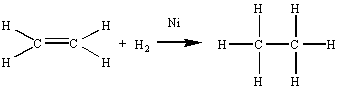
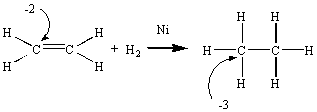
No comments:
Post a Comment