Liquid-Liquid Extraction
Extraction is a technique used for separating a compound from a mixture. An example is separating a water-insoluble organic compound from an aqueous mixture by extracting it into a water-insoluble organic solvent. These extractions are often part of the workup procedure for isolating and purifying the product of an organic reaction. Because trace amounts of water are often present at the end of an extraction process, a drying reagent is needed to ensure a dry product. The process of liquid-liquid extraction involves the distribution of a compound between two solvents that are insoluble in each other. By taking advantage of the differing solubilities of a solute in a pair of solvents, compounds can be selectively transported from one liquid phase to the other.This occurrence is quantified by the partition coefficient (K):
Liquid-Liquid Extraction FlowChart
Here is an example of a simple liquid-liquid extraction problem.
You want to isolate a mixture of compounds, and you do this by taking advantage of the bascicity and acidity of certain compounds. It is also important to note those structures that are rather neutral. Some key concepts are, bases will react with acid and vice versa. Also, the aqueous layer will contain a charged molecule, or ion, most of the time.
Step 1: Upon dissolving the mixture in a good solvent such as methylene chloride, a strong acid is added to the mixture.
The strong acid is added so that it will react with any basic compounds in the mixture, which wil lead it into the aqueous layer. Thus, one of the compounds has been separated. Adding base will deprotonate the proton from the nitrogen and give you the isolated product in the organic layer.
Step 2: To the remaining 3 compounds, a weak base is added, such as sodium carbonate.
The weak base will deprotonate the most acidic of the hydrogens, which is in this case, the carboxylic acid. The carboxylic acid containing structuer will be separated into the aqueous layer. Keep in mind, that the base is not limited to only deprotonating one structure, but this all just depends on the acidity of the compounds.
Step 3: To the final 2 compounds, a stronger base such as potassium hydroxide which will deprotonate the phenol.
Generally phenols are not very acidic, that is why a strong base must be used. The cyano containing compound is left in the organic layer.
Reaction Complete. All 4 compounds have been isolated into either aqueous or organic, and as mentioned eariler, it is very easy to bring an aqueous compound back into the organic, that is by adding the complementary acid or base
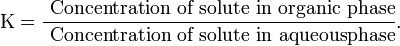
No comments:
Post a Comment