The alkylation of ammonia, Gabriel synthesis, reduction of nitriles,
reduction of amides, reduction of nitrocompounds, and reductive
amination of aldehydes and ketones are methods commonly used for
preparing amines.
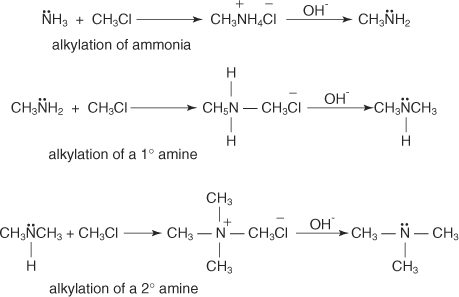
The reaction of ammonia with an alkyl halide leads to the formation
of a primary amine. The primary amine that is formed can also react
with the alkyl halide, which leads to a disubstituted amine that can
further react to form a trisubstituted amine. Therefore, the alkylation
of ammonia leads to a mixture of products.
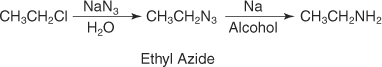
Reduction of alkylazides
You can best prepare a primary amine from its alkylazide by reduction or by the Gabriel synthesis.
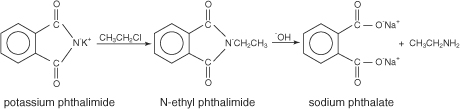
In the
Gabriel synthesis, potassium phthalimide is reacted
with an alkyl halide to produce an N-alkyl phthalimide. This N-alkyl
phthalimide can be hydrolyzed by aqueous acids or bases into the primary
amine.
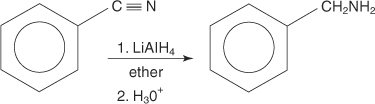
Reduction of nitriles
Nitriles can be reduced by lithium aluminum hydride (LiAIH4) to primary amines.
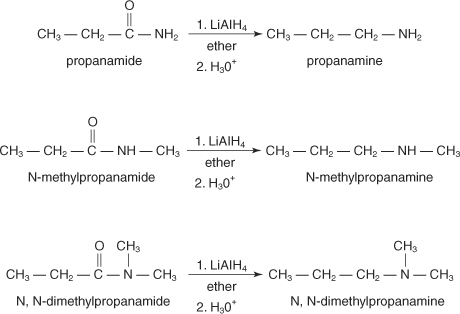
Reduction of amides
Amides yield primary amines on reduction by lithium aluminum
hydride, while N-substituted and N, N-disubstituted amides produce
secondary and tertiary amines, respectively.
Because amides are easily prepared, their reduction is a preferred method for making all classes of amines.
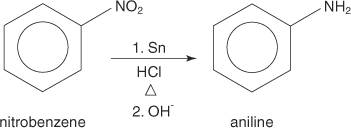
Reduction of nitrocompounds
Aromatic amines are normally prepared by reduction of the corresponding aromatic nitrocompound.
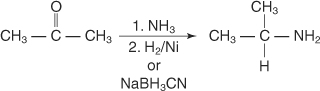
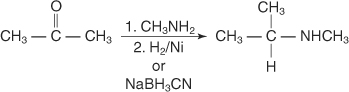
Reductive amination of aldehydes and ketones
Aldehydes or ketones can be reduced by catalytic or chemical
reductions in the presence of ammonia or primary or secondary amines,
producing primary, secondary, or tertiary amines.
The reaction of a ketone with ammonia, followed by catalytic
reduction or reduction by sodium cyanoborohydride, produces a 1° amine.
N-substituted amines are produced by reaction of ketones with primary amines, followed by reduction.
N,N-disubstituted amines can be produced by reaction of 2° amines with ketones followed by reduction
No comments:
Post a Comment