Cycloalkanes have one or more
rings of carbon atoms. The simplest examples of this class consist of a
single, unsubstituted carbon ring, and these form a homologous series similar
to the unbranched alkanes. The IUPAC names of the first five members of
this series are given in the following table. The last (yellow shaded) column gives
the general formula for a cycloalkane of any size. If a simple unbranched
alkane is converted to a cycloalkane two hydrogen atoms, one from each end
of the chain, must be lost. Hence the general formula for a cycloalkane
composed of n carbons is CnH2n.
Although a cycloalkane has two fewer hydrogens than the equivalent
alkane, each carbon is bonded to four other atoms so such compounds are
still considered to be saturated with hydrogen.
Substituted cycloalkanes are named in a fashion very similar to that
used for naming branched alkanes. The chief difference in the rules and
procedures occurs in the numbering system. Since all the carbons of a
ring are equivalent (a ring has no ends like a chain does),
the numbering starts at a substituted ring atom.
For examples of how these rules are used in naming substituted cycloalkanes .
Small rings, such as three and four membered rings, have significant angle strain resulting from the distortion of the sp3 carbon bond angles from the ideal 109.5º to 60º and 90º respectively. This angle strain often enhances the chemical reactivity of such compounds, leading to ring cleavage products. It is also important to recognize that, with the exception of cyclopropane, cycloalkyl rings are not planar (flat). The three dimensional shapes assumed by the common rings (especially cyclohexane and larger rings) are described and discussed in the Conformational Analysis Section.
Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with m rings the formula is: CnH(2n + 2 - 2m). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following
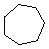
| Name | Cyclopropane | Cyclobutane | Cyclopentane | Cyclohexane | Cycloheptane | Cycloalkane |
|---|---|---|---|---|---|---|
| Molecular Formula |
C3H6 | C4H8 | C5H10 | C6H12 | C7H14 | CnH2n |
| Structural Formula |
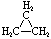 |
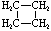 |
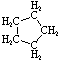 |
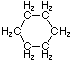 |
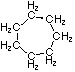 |
(CH2)n |
| Line Formula |
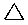 |
 |
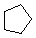 |
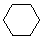 |
 |
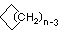 |
IUPAC Rules for Cycloalkane Nomenclature2. If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane. 3. If two different substituents are present on the ring, they are listed in alphabetical order, and the first cited substituent is assigned to carbon #1. The numbering of ring carbons then continues in a direction (clockwise or counter-clockwise) that affords the second substituent the lower possible location number. 4. If several substituents are present on the ring, they are listed in alphabetical order. Location numbers are assigned to the substituents so that one of them is at carbon #1 and the other locations have the lowest possible numbers, counting in either a clockwise or counter-clockwise direction. 5. The name is assembled, listing groups in alphabetical order and giving each group (if there are two or more) a location number. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. |
Small rings, such as three and four membered rings, have significant angle strain resulting from the distortion of the sp3 carbon bond angles from the ideal 109.5º to 60º and 90º respectively. This angle strain often enhances the chemical reactivity of such compounds, leading to ring cleavage products. It is also important to recognize that, with the exception of cyclopropane, cycloalkyl rings are not planar (flat). The three dimensional shapes assumed by the common rings (especially cyclohexane and larger rings) are described and discussed in the Conformational Analysis Section.
Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with m rings the formula is: CnH(2n + 2 - 2m). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following
If the products you look for are not in our catalog we would be pleased to offer our custom synthesis service. 1-benzyl-3-methylimidazolium
ReplyDelete