Throughout most of history the idea that matter is composed of minute
particles had languished as a philosophical abstraction known as atomism,
and no clear relation between these "atoms" and the chemical "elements"
had been established. This began to change in the early 1800's when the
development of balances that permitted reasonably precise measurements
of the weight changes associated with chemical reactions ushered in a
new and fruitful era of experimental chemistry. This resulted in the
recognition of several laws of chemical change that laid the groundwork for the atomic theory of matter.
 More importantly, as we will see later, this experimental result
tells us something very imporant about the mass of the oxygen atom
relative to that of the magnesium atom.
More importantly, as we will see later, this experimental result
tells us something very imporant about the mass of the oxygen atom
relative to that of the magnesium atom.
 Many
combinations of elements can react to form more than one compound. In
such cases, this law states that the weights of one element that combine
with a fixed weight of another of these elements are integer multiples
of one another.
Many
combinations of elements can react to form more than one compound. In
such cases, this law states that the weights of one element that combine
with a fixed weight of another of these elements are integer multiples
of one another.
It's easy to say this, but please make sure that you understand how it works. Nitrogen forms a very large number of oxides, five of which are shown here.
 If Nobel prizes had existed in the early 1800's, the English schoolteacher/meteorologist/chemist John Dalton
(1766-1844) would certainly have won one for showing how the
experimental information available at that time, as embodied in the laws
of chemical change that we have just described, are fully consistent
with the hypothesis that atoms are the smallest units of chemical
identity.
If Nobel prizes had existed in the early 1800's, the English schoolteacher/meteorologist/chemist John Dalton
(1766-1844) would certainly have won one for showing how the
experimental information available at that time, as embodied in the laws
of chemical change that we have just described, are fully consistent
with the hypothesis that atoms are the smallest units of chemical
identity.
These points of Dalton's atomic theory provided satisfactory explanations of all the laws of chemical change noted above:
Laws of chemical change
Recall that a "law", in the context of science, is just a relationship, discovered through experimentation, that is sufficiently well established to be regarded as beyond question for most practical purposes. Because it is the nature of scientists to question the "unquestionable", it occasionally happens that exceptions do arise, in which case the law must undergo appropriate modification.Conservation of mass-energy
This is usually considered the most fundamental of law of nature. It is also a good example of a law that had to be modified; it was known simply as Conservation of Mass until Einstein showed that energy and mass are interchangeable. However, the older term is perfectly acceptable within the field of ordinary chemistry in which energy changes are too small to have a measurable effect on mass relations.
Within the context of chemistry, conservation of mass can be thought of as "conservation of atoms".
Chemical change just shuffles them around into new arrangements.
Mass conservation had special significance in understanding
chemical changes involving gases, which were for some time not always
regarded as real matter at all. (Owing to their very small densities,
carrying out actual weight measurements on gases is quite difficult to
do, and was far beyond the capabilities of the early expirimenters.)
Thus when magnesium metal is burned in air, the weight of the solid
product always exceeds that of the original metal, implying that the
process is one in which the metal combines with what might have been
thought to be a "weightless" component of the air, which we now know to
be oxygen.Chemical change just shuffles them around into new arrangements.
Law of definite proportions
This law is also known as the law of constant composition. It states that the proportion by weight of the element present in any pure substance is always the same. This enables us to generalize the relationship we illustrated above.
Problem Example 1
How many kilograms of metallic magnesium could theoreticaly be
obtained by decomposing 0.400 kg of magnesium oxide into its elements?
Solution: The mass ratio of Mg to O in this compound is 1/1.66 = 0.602,
so 0.400 kg of the oxide contains (0.400 kg) x 0.602 = 0.241 kg of Mg.
Solution: The mass ratio of Mg to O in this compound is 1/1.66 = 0.602,
so 0.400 kg of the oxide contains (0.400 kg) x 0.602 = 0.241 kg of Mg.
The fact that we are concerned with the reverse of the reaction cited above is irrelevant.
The laws of definite and of multiple proportions are known collectively as the laws of chemical composition.
Law of multiple proportions
 Many
combinations of elements can react to form more than one compound. In
such cases, this law states that the weights of one element that combine
with a fixed weight of another of these elements are integer multiples
of one another.
Many
combinations of elements can react to form more than one compound. In
such cases, this law states that the weights of one element that combine
with a fixed weight of another of these elements are integer multiples
of one another. It's easy to say this, but please make sure that you understand how it works. Nitrogen forms a very large number of oxides, five of which are shown here.
- Line
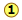 shows
the ratio of the relative weights of the two elements in each compound.
These ratios were calculated by simply taking the molar mass of each
element, and multiplying by the number of atoms of that element per mole
of the compound. Thus for NO2, we have (1 × 14) : (2 × 16) =
13:32. (These numbers were not known in the early days of Chemistry
because atomic weights (i.e., molar masses) of most elements were not
reliably known.)
shows
the ratio of the relative weights of the two elements in each compound.
These ratios were calculated by simply taking the molar mass of each
element, and multiplying by the number of atoms of that element per mole
of the compound. Thus for NO2, we have (1 × 14) : (2 × 16) =
13:32. (These numbers were not known in the early days of Chemistry
because atomic weights (i.e., molar masses) of most elements were not
reliably known.) - The numbers in Line
 are
just the mass ratios of O:N, found by dividing the corresponding ratios
in line 1. But someone who depends solely on experiment would work
these out by finding the mass of O that combines with unit mass (1 g) of
nitrogen.
are
just the mass ratios of O:N, found by dividing the corresponding ratios
in line 1. But someone who depends solely on experiment would work
these out by finding the mass of O that combines with unit mass (1 g) of
nitrogen. - Line is obtained by dividing the figures the previous line by the smallest O:N ratio in the line above, which is the one for N2O. Note that just as the law of multiple proportions says, the weight of oxygen that combines with unit weight of nitrogen work out to small integers.
- Of course we just as easily could have illustrated the law by considering the mass of nitrogen that combines with one gram of oxygen; it works both ways!
Problem Example 2
Nitrogen and hydrogen form many compounds, some of which
involve other elements as well. The mass of hydrogen that combines with
1.00 g of nitrogen to form three of these compounds are: urea, 0.1428 g;
ammonia, 0.0714 g; ammonium chloride, 0.2857 g. Show that this data is
consistent with the Law of Multiple Proportions.
Solution: The "fixed weight" we are considering here is the nitrogen. Inspection of the numbers above shows that the ammonia contains the smallest weight ratio H:N = 0.0714, while the weight ratio of H:N in urea is twice this number, and that in ammonium chloride is four times 0.0714. Thus the H:N ratios are themselves stand in the ratio of 2:1:4, respectively, and the Law is confirmed.
Solution: The "fixed weight" we are considering here is the nitrogen. Inspection of the numbers above shows that the ammonia contains the smallest weight ratio H:N = 0.0714, while the weight ratio of H:N in urea is twice this number, and that in ammonium chloride is four times 0.0714. Thus the H:N ratios are themselves stand in the ratio of 2:1:4, respectively, and the Law is confirmed.
How Dalton's interpretation of the laws of chemical change established the atomic theory
The idea that matter is composed of tiny "atoms" of some kind had been around for at least 2000 years. Dalton's accomplishment was to identify atoms with actual chemical elements.

These points of Dalton's atomic theory provided satisfactory explanations of all the laws of chemical change noted above:
Explanation of the law of conservation of mass
This is really a consequence of "conservation of atoms" which are presumed to be indestructible by chemical means. In chemical reactions, the atoms are simply rearranged, but never destroyed.
Explanation of the law of consant composition
If compounds are made up of definite numbers of atoms, each of which has its own characteristic mass, then the relative mass of each element in a compound must always be the same. Thus the elements must always be present in a pure sample of a compound in the same proportions by mass.
A given set of elements can usually form two or more compounds in which the numbers of atoms of some of the elements are different. Because these numbers must be integers (you can't have "half" an atom!), the mass of one element combined with a fixed mass of any other elements in any two such compounds can differ only by integer numbers. Thus, for the series of nitrogen-hydrogen compounds cited in the Problem Example above, we have the following relations:
| Compound | Formula | weight ratio H:N | ratio to 0.0714 |
|---|---|---|---|
urea
|
CO(NH2)2
|
0.1428
|
2
|
ammonia
|
NH3
|
0.0714
|
1
|
ammonium chloride
|
NH4Cl
|
0.2857
|
4
|
No comments:
Post a Comment