Grignard Reaction
Grignard Reagents

The Grignard Reaction is the addition of an organomagnesium halide (Grignard reagent) to a ketone or aldehyde, to form a tertiary or secondary alcohol, respectively. The reaction with formaldehyde leads to a primary alcohol.
Grignard Reagents are also used in the following important reactions: The addition of an excess of a Grignard reagent to an ester or lactone gives a tertiary alcohol in which two alkyl groups are the same, and the addition of a Grignard reagent to a nitrile produces an unsymmetrical ketone via a metalloimine intermediate. (Some more reactions are depicted below)
Mechanism of the Grignard Reaction
While the reaction is generally thought to proceed through a nucleophilic addition mechanism, sterically hindered substrates may react according to an SET (single electron transfer) mechanism: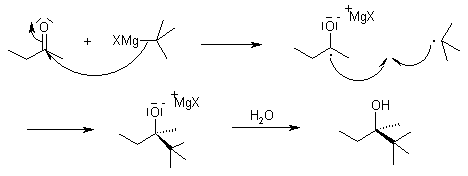
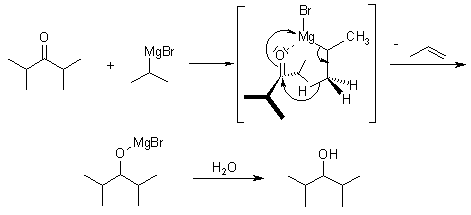
With sterically hindered ketones the following side products are received:
The Grignard reagent can act as base, with deprotonation yielding an enolate intermediate. After work up, the starting ketone is recovered.

A reduction can also take place, in which a hydride is delivered from the β-carbon of the Grignard reagent to the carbonyl carbon via a cyclic six-membered transition state.
Additional reactions of Grignard Reagents:
With carboxylic acid chlorides:

Esters are less reactive than the intermediate ketones, therefore the reaction is only suitable for synthesis of tertiary alcohols using an excess of Grignard Reagent:

With nitriles:

With CO2 (by adding dry ice to the reaction mixture
It’s arduous to seek out knowledgeable people on this matter, but you sound like you already know what you’re talking about! Thanksreagent
ReplyDelete