Imagine that a pair of crystallizing dishes are placed on an overhead projector as
shown in the figure below. An
alkene is added to the dish in the upper-left
corner of the projector and an
alkane is added to the dish in the upper-right
corner. A few drops of bromine dissolved in chloroform (CHCl
3) are then added
to each of the crystallizing dishes.
The characteristic red-orange color of bromine disappears the instant this reagent is
added to the alkene in the upper-left corner as the Br
2 molecules add across
the C=C double bond in the alkene.
The other crystallizing dish picks up the characteristic color of a dilute solution of
bromine because this reagent doesn't react with alkanes under normal conditions.
If the crystallizing dish in the upper-right corner is moved into the center of the
projector, however, the color of the bromine slowly disappears. This can be explained by
noting that alkanes react with halogens at high temperatures or in the presence of light
to form alkyl halides.
The light source in an overhead projector is intense enough to initiate this reaction,
although the reaction is still significantly slower than the addition of Br
2 to
an alkene.
The reaction between an alkane and one of the halogens (F
2, Cl
2,
Br
2, or I
2) can be understood by turning to a simpler example.
CH
4(
g) + Cl
2(
g)
CH
3Cl(
g) + HCl(
g)
This reaction has the following characteristic properties.
- It doesn't take place in the dark or at low temperatures.
- It occurs in the presence of ultraviolet light or at temperatures above 250ºC.
- Once the reaction gets started, it continues after the light is turned off.
- The products of the reaction include CH2Cl2 (dichloromethane),
CHCl3 (chloroform), and CCl4 (carbon tetrachloride), as well as CH3Cl
(chloromethane).
- The reaction also produces some C2H6.
These facts are consistent with a
chain-reaction mechanism that
involves three processes: chain initiation, chain propagation, and chain termination.
Chain Initiation
A Cl
2 molecule can dissociate into a pair of chlorine atoms by absorbing
energy in the form of either ultraviolet light or heat.
| Cl2 |
 |
2 Cl · |
|
|
 Ho = 243.4
kJ/molrxn Ho = 243.4
kJ/molrxn |
The chlorine atom produced in this reaction is an example of a
free radical

an atom or molecule that
contains one or more unpaired electrons.
Chain Propagation
Free radicals, such as the Cl· atom, are extremely reactive. When a chlorine atom
collides with a methane molecule, it can abstract a hydrogen atom to form HCl and a CH
3·
radical.
| CH4 + Cl· |
 |
CH3· + HCl |
|
|
 Ho = -16
kJ/molrxn Ho = -16
kJ/molrxn |
If the CH
3· radical then collides with a Cl
2 molecule, it can
remove a chlorine atom to form CH
3Cl and a new Cl· radical.
| CH3· + Cl2 |
 |
CH3Cl + Cl· |
|
|
 Ho = -87
kJ/molrxn Ho = -87
kJ/molrxn |
Because a Cl· atom is generated in the second reaction for every Cl· atom consumed in
the first, this reaction continues in a chain-like fashion until the radicals involved in
these chain-propagation steps are destroyed.
Chain Termination
If a pair of the radicals that keep the chain reaction going collide, they combine in a
chain-terminating step. Chain termination can occur in three ways.
| 2 Cl · |
 |
Cl2 |
|
|
 Ho = -243.4
kJ/molrxn Ho = -243.4
kJ/molrxn |
| CH3· + Cl · |
 |
CH3Cl |
|
|
 Ho = -330
kJ/molrxn Ho = -330
kJ/molrxn |
| 2 CH3· |
 |
CH3CH3 |
|
|
 Ho = -350
kJ/molrxn Ho = -350
kJ/molrxn |
Because the concentration of the radicals is relatively small, these chain-termination
reactions are relatively infrequent.
This chain-reaction mechanism for free-radical reactions explains the observations
listed for the reaction between CH
4 and Cl
2.
- The reaction doesn't occur in the dark or at low temperatures because energy must be
absorbed to generate the free radicals that carry the reaction.
| Cl2 |
 |
2 Cl· |
|
|
 Ho = 243.4
kJ/molrxn Ho = 243.4
kJ/molrxn |
- The reaction occurs in the presence of ultraviolet light because a UV photon has enough
energy to dissociate a Cl2 molecule to a pair of Cl· atoms. The reaction
occurs at high temperatures because Cl2 molecules can dissociate to form Cl·
atoms by absorbing thermal energy.
- The reaction continues after the light has been turned off because light is only needed
to generate the Cl· atoms that start the reaction. The chain reaction then converts CH4
into CH3Cl without consuming these Cl· atoms.
| CH4 + Cl· |
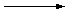 |
CH3· + HCl |
| CH3· + Cl2 |
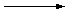 |
CH3Cl + Cl· |
- The reaction doesn't stop at CH3Cl because the Cl· atoms can abstract
additional hydrogen atoms to form CH2Cl2, CHCl3, and
eventually CCl4.
| CH3Cl + Cl· |
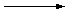 |
CH2Cl· + HCl |
|
| CH2Cl· + Cl2 |
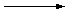 |
CH2Cl2 + Cl·, |
and so on |
- The formation of C2H6 is a clear indication that the reaction
proceeds through a free-radical mechanism. When two CH3· radicals collide,
they combine to form a ethane molecule.
| 2 CH3· |
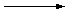 |
CH3CH3 |
Free-radical halogenation of alkanes provides us with another example of the role of
atom-transfer reactions in organic chemistry. The net effect of this reaction is to
oxidize a carbon atom by removing a hydrogen from this atom.
| CH4 |
+ |
Cl2 |
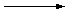 |
CH3Cl |
+ |
HCl |
| -4 |
|
|
|
-2 |
|
|
The reaction, however, doesn't involve the gain or loss of electrons. It occurs by the
transfer of a hydrogen atom in one direction and a chlorine atom in the other
 The characteristic red-orange color of bromine disappears the instant this reagent is
added to the alkene in the upper-left corner as the Br2 molecules add across
the C=C double bond in the alkene.
The characteristic red-orange color of bromine disappears the instant this reagent is
added to the alkene in the upper-left corner as the Br2 molecules add across
the C=C double bond in the alkene.  The other crystallizing dish picks up the characteristic color of a dilute solution of
bromine because this reagent doesn't react with alkanes under normal conditions.
The other crystallizing dish picks up the characteristic color of a dilute solution of
bromine because this reagent doesn't react with alkanes under normal conditions.  The light source in an overhead projector is intense enough to initiate this reaction,
although the reaction is still significantly slower than the addition of Br2 to
an alkene.
The light source in an overhead projector is intense enough to initiate this reaction,
although the reaction is still significantly slower than the addition of Br2 to
an alkene.  an atom or molecule that
contains one or more unpaired electrons.
an atom or molecule that
contains one or more unpaired electrons. 

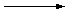 CH3Cl(g) + HCl(g)
CH3Cl(g) + HCl(g)
No comments:
Post a Comment