Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes,
depending on whether the carbon atoms of the molecule are arranged only
in chains or also in rings. Although these hydrocarbons have no
functional groups, they constitute the framework on which functional
groups are located in other classes of compounds, and provide an ideal
starting point for studying and naming organic compounds. The alkanes
and cycloalkanes are also members of a larger class of compounds
referred to as aliphatic. Simply put, aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure.
The following table lists the IUPAC names assigned to simple continuous-chain alkanes from C-1 to C-10. A common "ane" suffix identifies these compounds as alkanes. Longer chain alkanes are well known, and their names may be found in many reference and text books. The names methane through decane should be memorized, since they constitute the root of many IUPAC names. Fortunately, common numerical prefixes are used in naming chains of five or more carbon atoms.
Some important behavior trends and terminologies:
(i) The formulas and structures of these alkanes increase uniformly
by a CH2 increment.
(ii) A uniform variation of this kind in a series of compounds is called homologous.
(iii) These formulas all fit the CnH2n+2 rule. This is also the highest possible H/C ratio for a stable hydrocarbon.
(iv) Since the H/C ratio in these compounds is at a maximum, we call them saturated (with hydrogen).
Beginning with butane (C4H10), and becoming more numerous with larger alkanes,
we note the existence of alkane isomers. For example, there are five C6H14
isomers, shown below as abbreviated line formulas (A through E):
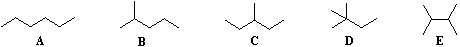 Although these distinct compounds all have the same molecular formula, only one (A) can be called hexane.
How then are we to name the others?
Although these distinct compounds all have the same molecular formula, only one (A) can be called hexane.
How then are we to name the others?
The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we have names for simple alkyl groups that may be attached to the chains. Examples of some common alkyl groups are given in the following table. Note that the "ane" suffix is replaced by "yl" in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group.
For the above isomers of hexane the IUPAC names are: B 2-methylpentane C 3-methylpentane
D 2,2-dimethylbutane E 2,3-dimethylbutane
Halogen substituents are easily accommodated, using the names: fluoro (F-), chloro (Cl-), bromo (Br-) and iodo (I-). For example, (CH3)2CHCH2CH2Br
would be named 1-bromo-3-methylbutane. If the halogen is bonded to a
simple alkyl group an alternative "alkyl halide" name may be used. Thus,
C2H5Cl may be named chloroethane (no locator
number is needed for a two carbon chain) or ethyl chloride. Halogenated
alkyl substituents such as bromomethyl, BrCH2–, and trichloromethyl, CCl3–, may be listed and are alphabetized according to their full name
The following table lists the IUPAC names assigned to simple continuous-chain alkanes from C-1 to C-10. A common "ane" suffix identifies these compounds as alkanes. Longer chain alkanes are well known, and their names may be found in many reference and text books. The names methane through decane should be memorized, since they constitute the root of many IUPAC names. Fortunately, common numerical prefixes are used in naming chains of five or more carbon atoms.
| Name | Molecular Formula | Structural Formula | Isomers | Name | Molecular Formula | Structural Formula | Isomers | |
|---|---|---|---|---|---|---|---|---|
| methane | CH4 | CH4 | 1 | hexane | C6H14 | CH3(CH2)4CH3 | 5 | |
| ethane | C2H6 | CH3CH3 | 1 | heptane | C7H16 | CH3(CH2)5CH3 | 9 | |
| propane | C3H8 | CH3CH2CH3 | 1 | octane | C8H18 | CH3(CH2)6CH3 | 18 | |
| butane | C4H10 | CH3CH2CH2CH3 | 2 | nonane | C9H20 | CH3(CH2)7CH3 | 35 | |
| pentane | C5H12 | CH3(CH2)3CH3 | 3 | decane | C10H22 | CH3(CH2)8CH3 | 75 |
(ii) A uniform variation of this kind in a series of compounds is called homologous.
(iii) These formulas all fit the CnH2n+2 rule. This is also the highest possible H/C ratio for a stable hydrocarbon.
(iv) Since the H/C ratio in these compounds is at a maximum, we call them saturated (with hydrogen).
The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we have names for simple alkyl groups that may be attached to the chains. Examples of some common alkyl groups are given in the following table. Note that the "ane" suffix is replaced by "yl" in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group.
| Group | CH3– | C2H5– | CH3CH2CH2– | (CH3)2CH– | CH3CH2CH2CH2– | (CH3)2CHCH2– | CH3CH2CH(CH3)– | (CH3)3C– | R– |
| Name | Methyl | Ethyl | Propyl | Isopropyl | Butyl | Isobutyl | sec-Butyl | tert-Butyl | Alkyl |
IUPAC Rules for Alkane Nomenclature2. Identify and name groups attached to this chain. 3. Number the chain consecutively, starting at the end nearest a substituent group. 4. Designate the location of each substituent group by an appropriate number and name. 5. Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. |
No comments:
Post a Comment