Alkenes and alkynes are hydrocarbons which respectively have carbon-carbon double bond and carbon-carbon triple bond functional groups. The molecular formulas of these unsaturated hydrocarbons reflect the multiple bonding of the functional groups:
As noted earlier in the Analysis of Molecular Formulas
section, the molecular formula of a hydrocarbon provides information
about the possible structural types it may represent. For example,
consider compounds having the formula C5H8. The formula of the five-carbon alkane pentane is C5H12
so the difference in hydrogen content is 4. This difference suggests
such compounds may have a triple bond, two double bonds, a ring plus a
double bond, or two rings. Some examples are shown here, and there are
at least fourteen others!
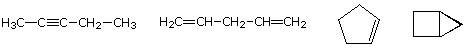
1. The yne suffix (ending) indicates an alkyne or cycloalkyne.
2. The longest chain chosen for the root name must include both carbon atoms of the triple bond.
3. The root chain must be numbered from the end nearest a triple bond carbon atom. If the triple bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts.
4. The smaller of the two numbers designating the carbon atoms of the triple bond is used as the triple bond locator.
5. If several multiple bonds are present, each must be assigned a locator number. Double bonds precede triple bonds in the IUPAC name, but the chain is numbered from the end nearest a multiple bond, regardless of its nature.
6. Because the triple bond is linear, it can only be accommodated in rings larger than ten carbons. In simple cycloalkynes the triple bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule.
7. Substituent groups containing triple bonds are:
HC≡C– Ethynyl group
HC≡CH–CH2– Propargyl group
| Alkane | R–CH2–CH2–R | CnH2n+2 | This is the maximum H/C ratio for a given number of carbon atoms. |
|---|---|---|---|
| Alkene | R–CH=CH–R | CnH2n | Each double bond reduces the number of hydrogen atoms by 2. |
| Alkyne | R–C≡C–R | CnH2n-2 | Each triple bond reduces the number of hydrogen atoms by 4. |
IUPAC Rules for Alkene and Cycloalkene Nomenclature2. The longest chain chosen for the root name must include both carbon atoms of the double bond. 3. The root chain must be numbered from the end nearest a double bond carbon atom. If the double bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts. 4. The smaller of the two numbers designating the carbon atoms of the double bond is used as the double bond locator. If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator number. 5. In cycloalkenes the double bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule. 6. Substituent groups containing double bonds are: H2C=CH– Vinyl group H2C=CH–CH2– Allyl group |
IUPAC Rules for Alkyne Nomenclature
2. The longest chain chosen for the root name must include both carbon atoms of the triple bond.
3. The root chain must be numbered from the end nearest a triple bond carbon atom. If the triple bond is in the center of the chain, the nearest substituent rule is used to determine the end where numbering starts.
4. The smaller of the two numbers designating the carbon atoms of the triple bond is used as the triple bond locator.
5. If several multiple bonds are present, each must be assigned a locator number. Double bonds precede triple bonds in the IUPAC name, but the chain is numbered from the end nearest a multiple bond, regardless of its nature.
6. Because the triple bond is linear, it can only be accommodated in rings larger than ten carbons. In simple cycloalkynes the triple bond carbons are assigned ring locations #1 and #2. Which of the two is #1 may be determined by the nearest substituent rule.
7. Substituent groups containing triple bonds are:
HC≡C– Ethynyl group
HC≡CH–CH2– Propargyl group
No comments:
Post a Comment