Acetoacetic Ester Synthesis
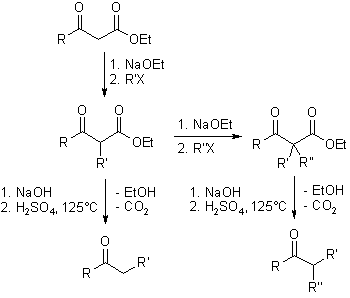
When α-keto acetic acid is treated with one mole of a base, the methylene group which is more acidic reacts with the base. And the reaction with an alkylation reagent gives alkyl products attached to methylene. When this reaction is repeated in the next step, the other hydrogen can also react to a dialkyl product. The two alkylation agents may be the same or different (R',R'').
β-Keto esters tend to decarboxylate after hydrolysation to β-keto carboxylic acid and heating to give one or two alkyl-substituted ketones, respectively.

If two equivalents of a strong base are added in the first step, the hydrogen of the more acidic methylene group, and in the next step the hydrogen of the methyl group (ambident nucleophiles), reacts with the base. The hydrogenated methyl group is, however, more acidic than the hydrogenated methylene group. The reaction with alkylation agent in the following step gives a product substituted at methyl group. This can be synthetically used to prepare selectively ketones of different types.
No comments:
Post a Comment