A
common problem encountered in chemistry involves the separation of a mixture of
two or three compounds into single compound fractions followed by the
purification and identification of each. To effect the separation, the chemist
must make use of the different properties of the components.
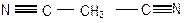
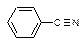
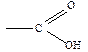
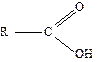
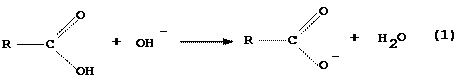
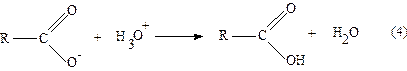
We
will use differences in solubility, density, acid-base chemistry and reactivity
to separate a mixture of compounds. We will then purify and identify each
component. The components will be unknown to the student except that one will
be a liquid neutral organic compound with a high boiling point and the other a
carboxylic acid. The carboxylic acid can react with a base such as sodium
hydroxide to form an anion which is water soluble. The neutral will not react
and so it will remain “neutral”. The possible organic neutral compounds are
listed below:
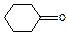
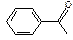
Table 3-1: Possible Neutral Unknown Compounds and Boiling Points
HYDROCARBONS
Decalin
190°C (2021) o-Xylene
144°C (2213)
ALCOHOLS
Cyclohexanol
160°C (2213) Benzyl
alcohol 205°C (2213)
KETONES
Cyclohexanone
157°C (2213) Acetophenone
200°C (1212)
NITRILES
Malononitrile
219°C (2223) Benzonitrile
191°C (1211)
Carboxylic
acids are compounds which include one or more carboxyl functional groups.
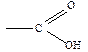
A
functional group can be defined as an atom or group of atoms in a definite
structural arrangement that influences the properties of an organic compound.
The carboxyl group gives the following possible unknowns their acidic
character.
TABLE 3-2 POSSIBLE
ACID UNKNOWNS
ORGANIC CARBOXYLIC
ACIDS
Compound Melting
Range (°C) Hazard
Code
o-Toluic Acid (2-Methylbenzoic acid) 103-105 1112
Azelaic Acid 106-107 0112
m-Anisic Acid (3-Methoxybenzoic acid) 105-107 1112
2-Phenoxypropionic acid 116-119 3112
Benzoic Acid 121-123 1213
Sebacic Acid 131-134 1113
Cinnamic Acid 132-135 2112
1-Naphthoic Acid 157-160 2112
Salicylic Acid 158-160 2112
p-Toluic Acid (4-Methylbenzoic acid) 180-182 1112
p-Anisic Acid (4-Methoxybenzoic acid) 182-185 1112
Once the unknown neutral and acid compounds have been
separated and purified they will then be identified, the neutral compound via
its infrared spectrum and the carboxylic acid via its melting range.
Extraction
The
general formula for a carboxylic acid is
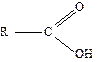
where
R stands for any group of atoms attached to the functional group COOH.
As with any Bronsted acid, a carboxylic acid reacts with hydroxide ion, OH-, to produce the conjugate base of the acid and water
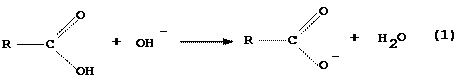
Carboxylic
acid Carboxylate anion
insoluble
in water soluble in water but
but
soluble in insoluble in organic
organic
liquids liquids
As
is indicated, this reaction also changes the solubility properties of the acid
molecule. We will take advantage of these property changes in separating the
acid from the mixture.
The
neutral component of the mixture may be any one of the hydrocarbons, alcohols,
ketones, or nitriles listed in Table 3-1. Neutral compounds will not react with
either an acid or a base. They are also water insoluble but very soluble in
organic liquids.
The Separation of the Aqueous and Organic
Layers
We
have now identified the solubility properties of the two components of the
mixture which will allow us to separate them. We will use liquid-liquid
extraction to take advantage of the differences in solubility of the
components. The organic liquid or solvent will be tert-butyl methyl ether
(TBME) and the polar aqueous layer will be 5% NaOH or water.
Initially
the mixture of the neutral organic and carboxylic acid unknowns will be
dissolved in TBME forming an organic solution. Suppose that this organic
solution is shaken with a dilute aqueous sodium hydroxide solution and then
allowed to stand until the two layers separate. During the shaking process,
the hydroxide ion
will
react with only the carboxylic acid 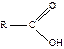 component of the mixture to form
the water soluble carboxylate anion (see Equation 1 again). This changes the
solubility properties of the acid as already stated and results in most of it
moving from the organic liquid layer to hydroxide ion-water layer. The
carboxylate ion thus is the solute and is extracted into the aqueous
(water) phase. The two phases are then separated into two fractions: The
aqueous sodium hydroxide solution containing the carboxylate anion as its salt
and the organic layer containing the neutral organic compound.
component of the mixture to form
the water soluble carboxylate anion (see Equation 1 again). This changes the
solubility properties of the acid as already stated and results in most of it
moving from the organic liquid layer to hydroxide ion-water layer. The
carboxylate ion thus is the solute and is extracted into the aqueous
(water) phase. The two phases are then separated into two fractions: The
aqueous sodium hydroxide solution containing the carboxylate anion as its salt
and the organic layer containing the neutral organic compound.
The
two phases will separate and form two separate layers based on differences in
polarity and density. The organic layer is much less polar and has a much
lower density compared to the diluted NaOH solution. Sometimes the difference
in polarity and/or density of the two phases may not be great enough to effect
a separation causing the formation of an emulsion. The separation can sometimes
be improved by adding more NaOH solution or TBME solvent.
It
is important to note that single extractions do not necessarily yield complete
separations, and that multiple extractions may be needed. In your work, you
will extract the original organic solution two times with aqueous sodium
hydroxide solution to remove the acid and water soluble impurities from the
organic layer. The two aqueous extracts are then combined and set aside as the
aqueous sodium hydroxide fraction. The organic solution is further extracted
once with distilled water to remove any water soluble impurities. Once these
extractions are complete, the organic solution should contain only the
"neutral" compound and the organic acid unknown should be extracted
into the NaOH solution.
Purification
Once
the two components have been separated, we must obtain each of them in a pure
form so they may be easily identified. The pure carboxylic acid in this
sequence is a solid while the neutral compound is a liquid. The water soluble
carboxylate anion will be precipitated from the sodium hydroxide extract by
adding 6M HCl and then recrystallized for purification. The pure neutral
compound can be obtained by distilling the lower boiling solvent (TBME) off,
leaving only the pure neutral liquid.
Precipitation
of the Acid
The
carboxylic acid is extracted into an aqueous hydroxide solution because the
carboxylate anion RCOO- dissolves more readily in water than in the
organic solvent because the anion is SOLVATED by the polar molecules. What
would happen if hydrochloric acid, HCl, were added to this carboxylate anion
fraction? The hydroxide ion would first react:
OH- + H3O+
® 2 H2O
and
then after the hydroxide ion has been consumed, the carboxylate anion would
react to yield the original acid
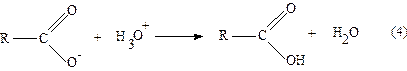
Since
the carboxylic acid has a very limited solubility in water most of it will
precipitate; that is, the solid will form in the solution. The solution is
then filtered to separate the acid crystals from the aqueous solvent, water.
In general, a solid which forms rapidly is not very pure because the crystal
lattice includes impurities and must be recrystallized for further
purification.
Purification
of the Neutral Compound
The
mixture of TBME and the neutral compound will next be treated with a drying
agent to remove any trace water before the TBME is removed by simple
distillation. The pure, neutral compound in this experiment is a liquid which
has a characteristic boiling point significantly higher than that of the
organic solvent, TBME which has a boiling point of 55°C and density 0.74 g/ml. Simple
distillation will then be used to remove the TBME from the neutral compound.
The TBME collected will be recycled. A "rotary evaporator" will then
be used to remove the final traces of TBME from the neutral organic compound.
The rotary evaporator is used to conduct a form of vacuum distillation and can
be used whenever the compounds which need to be separated have very different
boiling points. The organic solvent evaporates easily when heated at the low
pressure, leaving the neutral compound behind.
Identification
The acid in the unknown will be identified by
melting point and mixed melting points while the neutral compound will be
identified by infrared analysis. Read and study the information in the appendix
on these two techniques.
EXPERIMENTAL
At
first, it is remarkably easy to get lost in this sequence! The flow chart on
the next page is designed to help you get an overview of the procedure to help
keep track of where you are and where you are heading. If you do get lost hug
the nearest tree until you are found (just kidding).
PRELAB
ASSIGNMENT:
Review information on acid-base concepts, solvation, extraction,
recrystallization, simple distillation, infrared spectroscopy, melting temperature
determinations, drying agents in this experiment, the appendix, previous
experiments, and the lecture text. Then answer all of the pre-lab questions at
the end of this experiment
Alfa Chemistry employs more than 200 full time staff, of which approximate 80 are Ph.D. and M.S. chemists, specialized in synthetic chemistry, process optimization, and research. 1-octyl-3-methylimidazolium tosylate
ReplyDelete