Atoms and elements: what's the difference?
The parallel concepts of the element and the atom constitute the very foundations of chemical science.
Sulfur the element
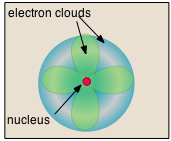
Sulfur the atom
The atom is the microscopic realization of this concept; that is, it is the actual physical particle that is unique to each chemical element. Their very small size has long prevented atoms from being observable by direct means, so their existence was not universally accepted until the late 19th Century. The fact that we still hear mention of the "atomic theory of matter" should not imply that there is now any doubt about the existence of atoms. Few theories in the history of science have been as thorougly validated and are as well understood.
Although the word atom usually refers to a specific kind of particle (an "atom of magnesium", for example), our everyday use of element tends to be more general, referring not only to a substance composed of a particular type of atom ("bromine is one of the few elements that are liquids at room temperature"), but also to atoms in a collective sense ("magnesium is one of the elements having two electrons in its outer shell").
Atoms are not new!
 The underlying concept of atoms as the basic building blocks of matter has been around for a long time.
The underlying concept of atoms as the basic building blocks of matter has been around for a long time.As early as 600 BCE, the Gujarati (Indian) philosopher Acharya Kanad wrote that "Every object of creation is made of atoms which in turn connect with each other to form molecules".
A couple of centuries later in 460 BCE, the Greek philosopher Democritus reasoned that if you keep breaking a piece of matter into smaller and smaller fragments, there will be some point at which the pieces cannot be made any smaller. He called these "basic matter particles"— in other words, atoms. But this was just philosophy; it would not become science until 1800 when John Dalton showed how the atomic concept followed naturally from the results of quantitative experiments based on weight measurements.
No comments:
Post a Comment