In sp2 hybridization, there is intermixing of one 2s and
two of the 2p orbitals of carbon in the excited state to form three
hybrid orbitals. These are oriented in trigonal planar geometry. Each sp2
hybrid orbital is occupied by one electron. The remaining pure 2p
orbital with one electron lies at right angle to the plane of hybrid
orbitals.
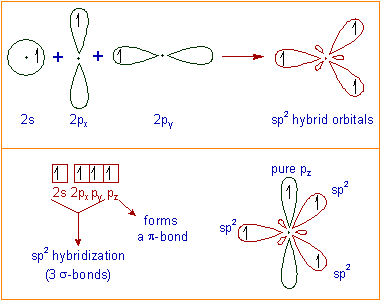 The sp2 hybrid orbital form 3 σ-bonds in trigonal planar geometry. Thus the bond angles are about 120o. The remaining pure 'p' orbital will form a π-bond. Thus carbon forms total four bonds i.e., three σ-bonds and one π-bond.
The sp2 hybrid orbital form 3 σ-bonds in trigonal planar geometry. Thus the bond angles are about 120o. The remaining pure 'p' orbital will form a π-bond. Thus carbon forms total four bonds i.e., three σ-bonds and one π-bond.
E.g. In ethylene molecule, C2H4, each carbon atom undergoes sp2 hybridization. Each carbon forms 2 σ-bonds with hydrogens and one σ-bond with another carbon. The remaining pure 'p' orbitals on two carbons overlap sidewise to form a π-bond. Thus there is a double bond between two carbons.
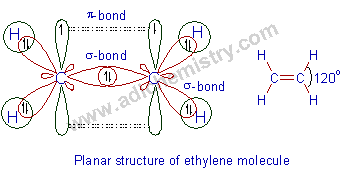 Note that whenever carbon atom undergoes sp2 hybridization, it forms 3 σ-bonds and 1 π-bond i.e., two single bonds and one double bond.
Note that whenever carbon atom undergoes sp2 hybridization, it forms 3 σ-bonds and 1 π-bond i.e., two single bonds and one double bond.

E.g. In ethylene molecule, C2H4, each carbon atom undergoes sp2 hybridization. Each carbon forms 2 σ-bonds with hydrogens and one σ-bond with another carbon. The remaining pure 'p' orbitals on two carbons overlap sidewise to form a π-bond. Thus there is a double bond between two carbons.

No comments:
Post a Comment