Let us start with the question "What is Organic chemistry?".
The simple answer is: It is the chemistry of carbon containing compounds, which are otherwise known as organic compounds.
So it is pretty easy to recognize that we should start our journey of organic chemistry by exploring the chemical nature of carbon.
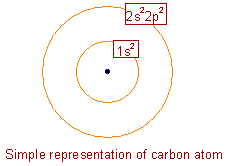
* Carbon is an element with atomic number (Z) = 6.
* Its ground state electronic configuration can be represented as: 1s22s22p2 (or) 1s22s22px12py12pz0
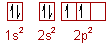 * It is the first element in Group-14 of Long form of
Periodic table.
* It is the first element in Group-14 of Long form of
Periodic table.
* It is a non metal.
* On Pauling's scale, its electronegativity value is around 2.5.
* It usually forms covalent bonds.
* Its valency is 4 since there are four electrons in the outer shell i.e., it can form four covalent bonds with other atoms.
Examples of organic compounds include carbohydrates, proteins, enzymes, vitamins, lipids, nucleic acids , synthetic polymers, synthetic fabrics, synthetic rubbers, plastics, medicines, drugs, organic dyes and so on.
Now the immediate question is: Why the carbon atom is so special and forms millions of compounds?
To answer this question, we should know about catenation.
Catenation is the ability of atoms of same element to bond covalently among themselves and form long chains or rings.
Carbon has a stronger tendency to catenate since it is a smaller atom and can form stronger covalent bonds with other carbons. The C-C bonds are stronger due to effective overlapping of atomic orbitals.
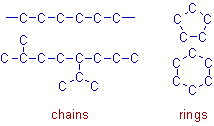 It also forms stronger bonds with other elements like hydrogen, oxygen, nitrogen, halogens, sulfur, phosphorus etc.
It also forms stronger bonds with other elements like hydrogen, oxygen, nitrogen, halogens, sulfur, phosphorus etc.
The organic compounds can also exhibit isomerism due to different structural and spatial arrangement of atoms or groups leading to formation of huge array of compounds.
These arguments explain why the carbon can form millions of compounds and organic chemistry is flourishing like nothing.
It is possible to understand the bonding in carbon compounds by using Lewi's dot model. According to this model, each atom participating in the bonding contributes one electron to form an electron pair which is shared between the two contributing atoms. Thus a covalent bond is formed. If atoms share two electron pairs, a double bond is formed. And a triple bond is formed when three electron pairs are shared.
The purpose of participating in bond formation is to get the nearest inert gas configuration and thus by getting stability. Most of the atoms try to get eight electrons or octet configuration in the valence shell. This is also called as octet rule.
The structures of some simple organic molecules are explained as shown below.
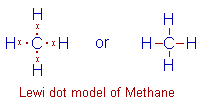
Methane, CH4: The carbon atom contributes four valence electrons to make four bonds with hydrogen atoms. Each hydrogen also contributes one electron for the bond formation.
Thus there are 4 C-H bonds in the methane molecule and carbon gets octet configuration in the valence shell.
Note that the valency of hydrogen atom is one. It can form only one bond since there is only one electron in this atom. It also gets Helium's configuration during bond formation.
Also note that in Lewi dot models, only the valence electrons are shown. The bond pairs can also be shown by lines.
 Ethane, C2H6: In Ethane molecule each carbon forms 4 bonds again. Among them three are C-H bonds, while the fourth one is a C-C bond.
Ethane, C2H6: In Ethane molecule each carbon forms 4 bonds again. Among them three are C-H bonds, while the fourth one is a C-C bond.
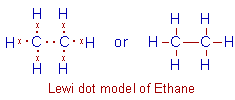 Ethylene, C2H4: In this molecule, there
is a double bond between two carbon atoms due to sharing of two pairs of
electrons. Each carbon also forms two bonds with hydrogen atoms.
Ethylene, C2H4: In this molecule, there
is a double bond between two carbon atoms due to sharing of two pairs of
electrons. Each carbon also forms two bonds with hydrogen atoms.
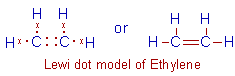 Acetylene, C2H2: There is a triple bond
between two carbon atoms in acetylene molecule. It is formed due to
sharing of three electron pairs. Each carbon also forms a single bond
with hydrogen atom.
Acetylene, C2H2: There is a triple bond
between two carbon atoms in acetylene molecule. It is formed due to
sharing of three electron pairs. Each carbon also forms a single bond
with hydrogen atom.
 Methyl fluoride, CH3F: Since there are 7 electrons
in the valence shell of Fluorine, it require one electron to complete
octet. Hence it contributes one electron for bond formation with carbon
as shown below.
Methyl fluoride, CH3F: Since there are 7 electrons
in the valence shell of Fluorine, it require one electron to complete
octet. Hence it contributes one electron for bond formation with carbon
as shown below.
Note that the only the bond pairs are shown as lines in the second representation.
There are three lone pairs and one bond pair around fluorine atom. The bond pair is shown as a line.
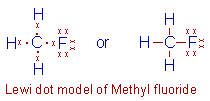 In the same way, carbon atom forms bonds with other halogen atoms.
In the same way, carbon atom forms bonds with other halogen atoms.
Formaldehyde, CH2O: There are six electrons in the valence shell of oxygen. It forms two bonds by contributing two of its valence electrons and thus by completing the octet. In formaldehyde, the oxygen atom forms two bonds with a carbon atom.
 Methyl alcohol, CH3OH: However, the oxygen atom can also
form just one bond with the carbon as in case of methyl alcohol. It forms the
second bond with hydrogen.
Methyl alcohol, CH3OH: However, the oxygen atom can also
form just one bond with the carbon as in case of methyl alcohol. It forms the
second bond with hydrogen.
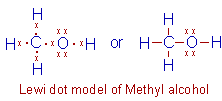 Hydrogen cyanide, HCN: The carbon atom can also form bonds with
nitrogen. In hydrogen cyanide there is a triple bond between carbon and
nitrogen. The nitrogen atom contributes three of its valence electrons for the
formation of this triple bond.
Hydrogen cyanide, HCN: The carbon atom can also form bonds with
nitrogen. In hydrogen cyanide there is a triple bond between carbon and
nitrogen. The nitrogen atom contributes three of its valence electrons for the
formation of this triple bond.
 Methyl amine, CH3NH2: In this molecule, the
carbon and nitrogen atoms are sharing only one pair of electrons.
Methyl amine, CH3NH2: In this molecule, the
carbon and nitrogen atoms are sharing only one pair of electrons.
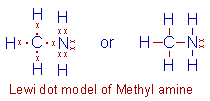 However this model could not explain the exact geometry of organic
molecules. For example, methane molecule is tetrahedral, whereas
ethylene is a planar molecule. These structures with exact bond angles
cannot be explained by this model. Therefore it is necessary to
understand the structures of these molecules by using valence bond
theory as explained in the next section.
However this model could not explain the exact geometry of organic
molecules. For example, methane molecule is tetrahedral, whereas
ethylene is a planar molecule. These structures with exact bond angles
cannot be explained by this model. Therefore it is necessary to
understand the structures of these molecules by using valence bond
theory as explained in the next section.
However it is possible to get 4 unpaired electrons by transferrin
The simple answer is: It is the chemistry of carbon containing compounds, which are otherwise known as organic compounds.
So it is pretty easy to recognize that we should start our journey of organic chemistry by exploring the chemical nature of carbon.
WHAT IS CARBON?
So the next question is: What is carbon?* Carbon is an element with atomic number (Z) = 6.
* Its ground state electronic configuration can be represented as: 1s22s22p2 (or) 1s22s22px12py12pz0
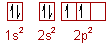
* It is a non metal.
* On Pauling's scale, its electronegativity value is around 2.5.
* It usually forms covalent bonds.
* Its valency is 4 since there are four electrons in the outer shell i.e., it can form four covalent bonds with other atoms.

WHY THERE ARE MILLIONS OF ORGANIC COMPOUNDS?
It is well known that there are millions of organic compounds around, which are either originated from the nature or prepared synthetically.Examples of organic compounds include carbohydrates, proteins, enzymes, vitamins, lipids, nucleic acids , synthetic polymers, synthetic fabrics, synthetic rubbers, plastics, medicines, drugs, organic dyes and so on.
Now the immediate question is: Why the carbon atom is so special and forms millions of compounds?
To answer this question, we should know about catenation.
Catenation is the ability of atoms of same element to bond covalently among themselves and form long chains or rings.
Carbon has a stronger tendency to catenate since it is a smaller atom and can form stronger covalent bonds with other carbons. The C-C bonds are stronger due to effective overlapping of atomic orbitals.
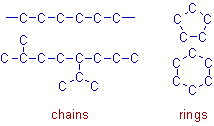
The organic compounds can also exhibit isomerism due to different structural and spatial arrangement of atoms or groups leading to formation of huge array of compounds.
These arguments explain why the carbon can form millions of compounds and organic chemistry is flourishing like nothing.
HOW DOES CARBON FORM CHEMICAL BONDS?
LEWI'S DOT MODEL
Carbon is an appreciably electronegative element and tends to form four covalent bonds by using all the four electrons in its valence shell i.e., the second shell for which the electronic configuration can be written as 2s22p2. Hence the combining power or the valency of carbon is 4. It can form 4 bonds with other atoms.It is possible to understand the bonding in carbon compounds by using Lewi's dot model. According to this model, each atom participating in the bonding contributes one electron to form an electron pair which is shared between the two contributing atoms. Thus a covalent bond is formed. If atoms share two electron pairs, a double bond is formed. And a triple bond is formed when three electron pairs are shared.
The purpose of participating in bond formation is to get the nearest inert gas configuration and thus by getting stability. Most of the atoms try to get eight electrons or octet configuration in the valence shell. This is also called as octet rule.
The structures of some simple organic molecules are explained as shown below.
Methane, CH4: The carbon atom contributes four valence electrons to make four bonds with hydrogen atoms. Each hydrogen also contributes one electron for the bond formation.
Thus there are 4 C-H bonds in the methane molecule and carbon gets octet configuration in the valence shell.
Note that the valency of hydrogen atom is one. It can form only one bond since there is only one electron in this atom. It also gets Helium's configuration during bond formation.
Also note that in Lewi dot models, only the valence electrons are shown. The bond pairs can also be shown by lines.




Note that the only the bond pairs are shown as lines in the second representation.
There are three lone pairs and one bond pair around fluorine atom. The bond pair is shown as a line.
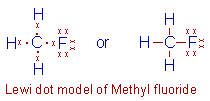
Formaldehyde, CH2O: There are six electrons in the valence shell of oxygen. It forms two bonds by contributing two of its valence electrons and thus by completing the octet. In formaldehyde, the oxygen atom forms two bonds with a carbon atom.

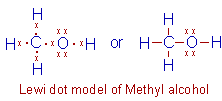

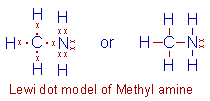
VALENCE BOND THEORY
According to valence bond theory, four unpaired electrons are required to form four covalent bonds. But there are only 2 unpaired electrons in the valence shell of carbon in the ground state.However it is possible to get 4 unpaired electrons by transferrin
No comments:
Post a Comment