|
This page looks briefly at how the chemistry of the "hydroxides" of
the Period 3 elements from sodium to chlorine varies as you cross the
period. I am taking the word "hydroxide" to include anything which contains either a hydroxide ion or an -OH group covalently bound to the element in question. You wouldn't usually think of some of the compounds on this page as hydroxides at all. A quick summary of the trends Sodium and magnesium hydroxides These contain hydroxide ions, and are simple basic hydroxides. Aluminium hydroxide Aluminium hydroxide, like aluminium oxide, is amphoteric - it has both basic and acidic properties. | |
|
Note: I have no real idea of how to describe aluminium hydroxide on the ionic-covalent spectrum. Part of the problem is that there are several forms of aluminium hydroxide. For example, when it is prepared by adding ammonia solution to a solution containing hexaaquaaluminium ions, [Al(H2O)6]3+, it is probably first formed as the covalently bound Al(H2O)3(OH)3. But this will lose water and rearrange itself on standing - and I don't know what exactly is formed. The chemistry textbooks that I have to hand aren't too clear about the structure of aluminium hydroxide as far as the degree of covalent character is concerened, and a web search (until I got totally bored with it!) didn't throw up any reliable chemistry sites which discussed it. Several geology sites describe the mineral gibbsite (a naturally occurring form of aluminium hydroxide) in terms of ions, but whether it actually contains ions or whether this is just a simplification as a convenient way of talking about and drawing a complicated structure, I don't know. | |
|
The other "hydroxides" In all of these have -OH groups covalently bound to the atom from period 3. These compounds are all acidic - ranging from the very weakly acidic silicic acids (one of which is shown below) to the very strong sulphuric or chloric(VII) acids. 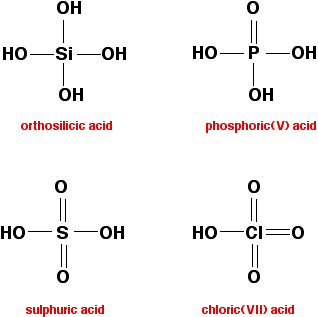 Adding some detail Sodium and magnesium hydroxides These are both basic because they contain hydroxide ions - a strong base. Both react with acids to form salts. For example, with dilute hydrochloric acid, you get colourless solutions of sodium chloride or magnesium chloride. Aluminium hydroxide Aluminium hydroxide is amphoteric. Like sodium or magnesium hydroxides, it will react with acids. This is showing the basic side of its nature. With dilute hydrochloric acid, a colourless solution of aluminium chloride is formed. But aluminium hydroxide also has an acidic side to its nature. It will react with sodium hydroxide solution to give a colourless solution of sodium tetrahydroxoaluminate. | |
|
Note: You may find all sorts of other formulae given for the product from this reaction. These range from NaAlO2 (which is a dehydrated form of the one in the equation) to Na3Al(OH)6 (which is a different product altogether). What you actually get will depend on things like the temperature and the concentration of the sodium hydroxide solution. In any case, the truth is almost certainly a lot more complicated than any of these. (That's equally true of the previous equation involving the acid.) This is a case where it is a good idea to find out what your examiners quote in their support material or mark schemes, and stick with that. If necessary, get this sort of information from your Exam Board by following the links on the syllabuses page. | |
|
The other "hydroxides" A quick reminder of what we are talking about here:  But they vary considerably in strength:
If the negative charge stays entirely on the oxygen atom left behind from the -OH group, it will be very attractive to hydrogen ions. The lost hydrogen ion will be easily recaptured and the acid will be weak. On the other hand, if the charge can be spread out (delocalised) over the whole of the ion, it will be so "dilute" that it won't attract the hydrogen back very easily. The acid will then be strong. Wherever possible, the negative charge is delocalised by interacting with doubly-bonded oxygens. For example, in chloric(VII) acid, the ion produced is the chlorate(VII) ion (also known as the perchlorate ion), ClO4-. The structure of the ion doesn't stay like this:  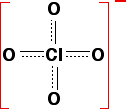 | |
|
Note: This is just like the delocalisation which occurs in the ethanoate ion formed when ethanoic acid is behaving as a weak acid (except on a larger scale). You will find this described in some detail on a page about organic acids. Use the BACK button on your browser if you choose to follow this link. | |
|
When sulphuric acid loses a hydrogen ion to form the hydrogensulphate ion, HSO4-,
the charge can be spread over three oxygens (the original one with the
negative charge, and the two sulphur-oxygen double bonds. That's still
an effective delocalisation, and sulphuric acid is almost as strong as
chloric(VII) acid. | |
|
Note: Sulphuric acid can, of course, lose a second hydrogen ion as well from the other -OH group and form sulphate ions. However, that is a bit more difficult. If you lose that second hydrogen, you can use all four oxygens to delocalise the charge - but now you have to delocalise two negative charges rather than just one. The hydrogensulphate ion isn't a strong acid. It's strength is similar to phosphoric(V) acid. | |
|
Phosphoric(V) acid is much weaker than sulphuric acid because it only
has one phosphorus-oxygen double bond which it can use to help
delocalise the charge on the ion formed by losing one hydrogen ion - so
the charge on that ion is delocalised less effectively. In orthosilicic acid, there aren't any silicon-oxygen double bonds to delocalise the charge. That means the ion formed by loss of a hydrogen ion isn't at all stable, and easily recovers its hydrogen | |
Saturday, 27 April 2013
PROPERTIES OF THE PERIOD 3 "HYDROXIDES"
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment