The first four saturated unbranched acyclic hydrocarbons are called
methane, ethane, propane and butane. Names of the higher members of this
series consist of
a numerical term, followed by "-ane" with elision of terminal "a" from
the numerical term. Examples of these names are shown in the table
below. The generic name of saturated acyclic hydrocarbons
(branched or unbranched) is "alkane".
Examples of names:
(n = total number of carbon atoms)
1.2 - Univalent radicals derived from saturated unbranched acyclic hydrocarbons by removal of hydrogen from a terminal carbon atom are named by replacing the ending "-ane" of the name of the hydrocarbon by "-yl". The carbon atom with the free valence is numbered as 1. As a class, these radicals are called normal, or unbranched chain, alkyls.
Examples to Rule A-1.2
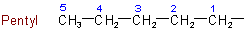
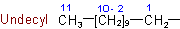
Examples of names:
(n = total number of carbon atoms)
1.2 - Univalent radicals derived from saturated unbranched acyclic hydrocarbons by removal of hydrogen from a terminal carbon atom are named by replacing the ending "-ane" of the name of the hydrocarbon by "-yl". The carbon atom with the free valence is numbered as 1. As a class, these radicals are called normal, or unbranched chain, alkyls.
Examples to Rule A-1.2
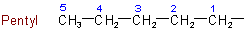
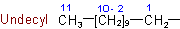

No comments:
Post a Comment