|
EQUILIBRIUM CONSTANTS: Kc
This page explains what is meant by an equilibrium constant,
introducing equilibrium constants expressed in terms of concentrations, Kc.
It assumes that you are familiar with the concept of a dynamic
equilibrium, and know what is meant by the terms "homogeneous" and
"heterogeneous" as applied to chemical reactions. | |
|
Important: If you aren't sure about dynamic equilibria it is important that you follow this link before you go on. If you aren't sure what homogeneous and heterogeneous mean, you would find it useful to follow this link and read the beginning of the page that you will find (actually on catalysis). Use the BACK button on your browser to return to this page. | |
We need to look at two different types of equilibria (homogeneous and
heterogeneous) separately, because the equilibrium constants are
defined differently.
A good example of a gaseous homogeneous equilibrium is the conversion of sulphur dioxide to sulphur trioxide at the heart of the Contact Process: A commonly used liquid example is the esterification reaction between an organic acid and an alcohol - for example: Writing an expression for Kc We are going to look at a general case with the equation: No state symbols have been given, but they will be all (g), or all (l), or all (aq) if the reaction was between substances in solution in water. If you allow this reaction to reach equilibrium and then measure the equilibrium concentrations of everything, you can combine these concentrations into an expression known as an equilibrium constant. The equilibrium constant always has the same value (provided you don't change the temperature), irrespective of the amounts of A, B, C and D you started with. It is also unaffected by a change in pressure or whether or not you are using a catalyst. 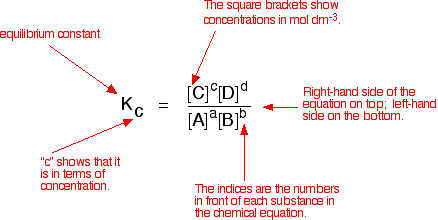 The indices (the powers that you have to raise the concentrations to - for example, squared or cubed or whatever) are just the numbers that appear in the equation. | |
|
Note: If you have come across orders of reaction, don't confuse this with the powers that appear in the rate equation for a reaction. Those powers (the order of the reaction with respect to each of the reactants) are experimentally determined. They don't have any direct connection with the numbers that appear in the equation You may come across attempts to derive the expression for Kc by writing rate equations for the forward and back reactions. Except in a very limited number of very simple examples, this can't be done! These attempts make the fundamental mistake of obtaining the rate equation from the chemical equation. That's WRONG! Deriving an expression for Kc is impossible at this level of chemistry. It isn't relevant to this page, but if you want to find out more about orders of reaction, you might like to follow this link at some time in the future. | |
|
Some specific examples The esterification reaction equilibrium A typical equation might be: There is only one molecule of everything shown in the equation. That means that all the powers in the equilibrium constant expression are "1". You don't need to write those into the Kc expression. 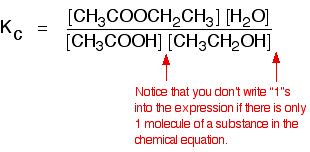 The equilibrium in the hydrolysis of esters This is the reverse of the last reaction: The Kc expression is: 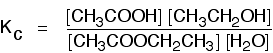 It is really important to write down the equilibrium reaction whenever you talk about an equilibrium constant. That is the only way that you can be sure that you have got the expression the right way up - with the right-hand substances on the top and the left-hand ones at the bottom. The Contact Process equilibrium You will remember that the equation for this is: This time the Kc expression will include some visible powers: 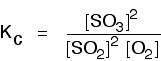 The Haber Process equilibrium The equation for this is: . . . and the Kc expression is: 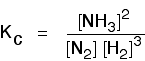 The equilibrium established if steam is in contact with red hot carbon. Here we have gases in contact with a solid. If you shake copper with silver nitrate solution, you get this equilibrium involving solids and aqueous ions: Writing an expression for Kc for a heterogeneous equilibrium The important difference this time is that you don't include any term for a solid in the equilibrium expression. Taking another look at the two examples above, and adding a third one: The equilibrium produced on heating carbon with steam Everything is exactly the same as before in the equilibrium constant expression, except that you leave out the solid carbon. 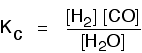 Both the copper on the left-hand side and the silver on the right are solids. Both are left out of the equilibrium constant expression. 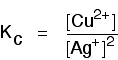 This equilibrium is only established if the calcium carbonate is heated in a closed system, preventing the carbon dioxide from escaping. The only thing in this equilibrium which isn't a solid is the carbon dioxide. That is all that is left in the equilibrium constant expression. This is simply too huge a topic to be able to deal with satisfactorily on the internet. It isn't the best medium for learning how to do chemistry calculations. It is much easier to do this from a carefully structured book giving you lots of worked examples and lots of problems to try yourself. If you have found this site useful, you might like to have a look at my book on chemistry calculations. It covers equilibrium constant calculations starting with the most trivial cases, and gradually getting harder - up to the moderately difficult examples which may be asked in a UK A' level examination. | |
Tuesday, 24 September 2013
EQUILIBRIUM CONSTANTS: Kc
Subscribe to:
Post Comments (Atom)
As a global Contract Research Organization (CRO), headquartered in New York, USA, Alfa Chemistry has served the pharmaceutical and biotechnology industries for eight years. 1-butyl-3-methylimidazolium tricyanomethane
ReplyDelete