|
CATALYSTS IN THE PETROCHEMICAL INDUSTRY
This page looks briefly at some of the basic processes in the
petrochemical industry (cracking, isomerisation and reforming) as
examples of important catalytic reactions. Catalytic cracking Cracking is the name given to breaking up large hydrocarbon molecules into smaller and more useful bits. This is achieved by using high pressures and temperatures without a catalyst, or lower temperatures and pressures in the presence of a catalyst. The source of the large hydrocarbon molecules is often the naphtha fraction or the gas oil fraction from the fractional distillation of crude oil (petroleum). These fractions are obtained from the distillation process as liquids, but are re-vaporised before cracking. The hydrocarbons are mixed with a very fine catalyst powder. These days the catalysts are zeolites (complex aluminosilicates) - these are more efficient than the older mixtures of aluminium oxide and silicon dioxide. The whole mixture is blown rather like a liquid through a reaction chamber at a temperature of about 500°C. Because the mixture behaves like a liquid, this is known as fluid catalytic cracking (or fluidised catalytic cracking). Although the mixture of gas and fine solid behaves as a liquid, this is nevertheless an example of heterogeneous catalysis - the catalyst is in a different phase from the reactants. | |
|
Note: If you don't understand the term heterogeneous catalysis, follow this link to the introductory page on catalysis. Use the BACK button on your browser to return quickly to this page. | |
|
The catalyst is recovered afterwards, and the cracked mixture is separated by cooling and further fractional distillation. There isn't any single unique reaction happening in the cracker. The hydrocarbon molecules are broken up in a fairly random way to produce mixtures of smaller hydrocarbons, some of which have carbon-carbon double bonds. One possible reaction involving the hydrocarbon C15H32 might be: 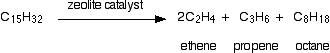 Or, showing more clearly what happens to the various atoms and bonds: 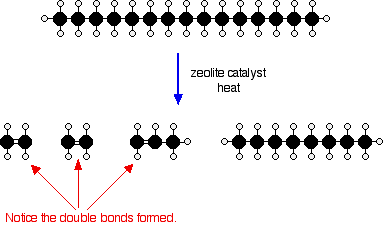 Isomerisation Hydrocarbons used in petrol (gasoline) are given an octane rating which relates to how effectively they perform in the engine. A hydrocarbon with a high octane rating burns more smoothly than one with a low octane rating. Molecules with "straight chains" have a tendency to pre-ignition. When the petrol / air mixture is compressed they tend to explode, and then explode a second time when the spark is passed through them. This double explosion produces knocking in the engine. | |
|
Note: A straight chain hydrocarbon isn't literally straight! It just means that all the carbon atoms are joined up one after another in a row. If you made a model it would be extremely bendy! | |
Octane ratings are based on a scale on which heptane is given a
rating of 0, and 2,2,4-trimethylpentane (an isomer of octane) a rating
of 100.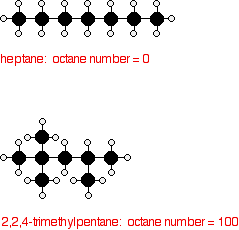 | |
|
Note: If you don't understand what is meant by the term structural isomer you really ought to follow this link before you go on. If you aren't happy about naming organic compounds, you might like to follow up this link at some time in the future. It isn't particularly important for the purposes of understanding the current page. Use the BACK button on your browser to return to this page. | |
|
In order to raise the octane rating of the molecules found in petrol
(gasoline) and so make the petrol burn better in modern engines, the oil
industry rearranges straight chain molecules into their isomers with
branched chains. One process uses a platinum catalyst on a zeolite base at a temperature of about 250°C and a pressure of 13 - 30 atmospheres. It is used particularly to change straight chains containing 5 or 6 carbon atoms into their branched isomers. For example: 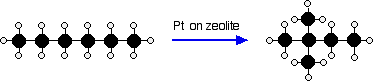 | |
|
Note: If you aren't familiar with the structure of benzene you might like to have a quick look at this link before you go on. There's no need to get too bogged down in it though for the purposes of the current page. Use the BACK button on your browser to return to this page. | |
|
Reforming uses a platinum catalyst suspended on aluminium oxide
together with various promoters to make the catalyst more efficient.
The original molecules are passed as vapours over the solid catalyst at a
temperature of about 500°C. Isomerisation reactions occur (as above) but, in addition, chain molecules get converted into rings with the loss of hydrogen. Hexane, for example, gets converted into benzene, and heptane into methylbenzene. 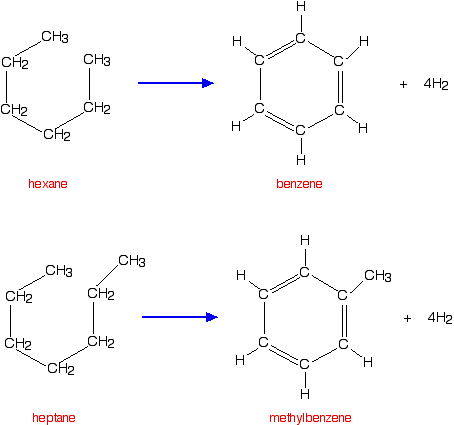 | |
|
Note: The Kekulé structure has been used for benzene because it is easier to understand if you aren't familiar with benzene chemistry. You could perfectly well use the standard symbol of a circle inside a hexagon instead. The original hexane and heptane molecules have been drawn bent into ring shapes so that you can easily see how they are changed during reforming. | |
Tuesday, 24 September 2013
CATALYSTS IN THE PETROCHEMICAL INDUSTRY
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment