|
ENERGY PROFILES FOR SIMPLE REACTIONS
This page takes a closer look at simple energy profiles for
reactions, and shows how they are slightly different for reactions
involving an intermediate or just a transition state. Both of those
terms are explained as well. Types of Energy Profile What is an energy profile? If you have done any work involving activation energy or catalysis, you will have come across diagrams like this:  It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the "activation energy barrier". In this example of a reaction profile, you can see that a catalyst offers a route for the reaction to follow which needs less activation energy. That, of course, causes the reaction to happen faster. | |
|
Note: If you aren't very happy about this, read the page about catalysts before you go on. Use the BACK button on your browser to return to this page, or come back via the rates of reaction menu. | |
|
Diagrams like this are described as energy profiles.
In the diagram above, you can clearly see that you need an input of
energy to get the reaction going. Once the activation energy barrier
has been passed, you can also see that you get even more energy
released, and so the reaction is overall exothermic. If you had an endothermic reaction, a simple energy profile for a non-catalysed reaction would look like this: 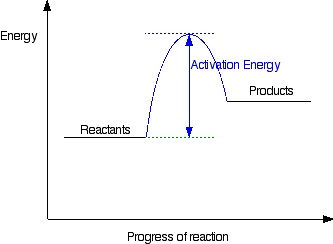 Energy profiles for reactions which go via a single transition state only This is much easier to talk about with a real example. The equation below shows an organic chemistry reaction in which a bromine atom is being replaced by an OH group in an organic compound. The starting compound is bromoethane, and the organic product is ethanol. During the reaction one of the lone pairs of electrons on the negatively charged oxygen in the -OH group is attracted to the carbon atom with the bromine attached. That's because the bromine is more electronegative than carbon, and so the electron pair in the C-Br bond is slightly closer to the bromine. The carbon atom becomes slightly positively charged and the bromine slightly negative. 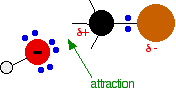 At some point, the process is exactly half complete. The carbon atom now has the oxygen half-attached, the bromine half-attached, and the three other groups still there, of course. 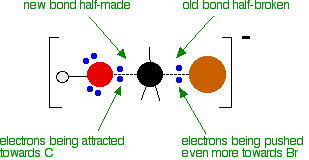 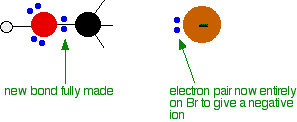 | |
|
Note: These diagrams have been simplified in various ways to make the process clearer. For example, the true arrangement of the lone pairs of electrons around the oxygen in the first diagram has been simplified for clarity. The bromine also has 3 lone pairs as well as the bonding pair, but they play no part. And, of course, the other groups attached to the carbon have been left out in order to concentrate on what is important. | |
The second diagram where the bonds are half-made and half-broken is called the transition state,
and it is at this point that the energy of the system is at its
maximum. This is what is at the top of the activation energy barrier.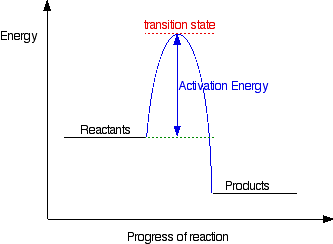 The situation is entirely different if the reaction goes through an intermediate. Again, we'll look at a specific example. Energy profiles for reactions which go via an intermediate For reasons which you may well meet in the organic chemistry part of your course, a different organic bromine-containing compound reacts with hydroxide ions in an entirely different way. In this case, the organic compound ionises slightly in a slow reaction to produce an intermediate positive organic ion. This then goes on to react very rapidly with hydroxide ions. 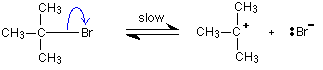 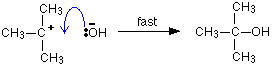 | |
|
Note: If you haven't come across the use of curly arrows in organic chemistry yet, all you need to know for now is that they show the movement of a pair of electrons. In the first equation, for example, the bonding pair of electrons in the C-Br bond moves entirely on to the bromine to make a bromide ion. In the second equation, a lone pair on the hydroxide ion moves towards the positive carbon to form a covalent bond. | |
The big difference in this case is that the positively charged
organic ion can actually be detected in the mixture. It is very
unstable, and soon reacts with a hydroxide ion (or picks up its bromide
ion again). But, for however short a time, it does have a real presence
in the system. That shows itself in the energy profile.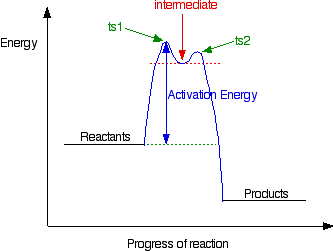 Notice that the barrier on the product side of the intermediate is lower than that on the reactant side. That means that there is a greater chance of it finding the extra bit of energy to convert into products. It would need a greater amount of energy to convert back to the reactants again. I've labelled these peaks "ts1" and "ts2" - they both represent transition states between the intermediate and either the reactants or the products. During either conversion, there will be some arrangement of the atoms which causes an energy maximum - that's all a transition state is. And finally . . . It is perfectly possible to get reactions which take several steps - going through a number of different intermediates and transition states. In cases like this, you would end up with a whole "mountain range" of peaks, some of which might be simple transition states, and others with the little dips which hold intermediates. You wouldn't expect to come across problems like this at levels equivalent to UK A level. | |
Tuesday, 24 September 2013
ENERGY PROFILES FOR SIMPLE REACTIONS
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment