Electrodes used in potentiometric
titrations
The
experimental setup for potentiometric measurement comprises
a set of an indicating and reference electrodes or two identical indicating
electrodes, which should be treated carefully. Do not place the electrodes
anywhere except attached to the electrode holder. At
the end of the experiment rinse the electrodes and place each one in its
housing as required.
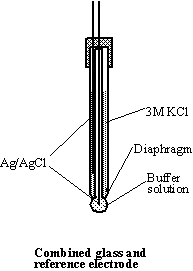
Glass electrode. Combined glass reference
electrode consists of indicator and reference electrodes in the same body.
Great care should be taken of it: never touch the glass part of the electrode
with anything except soft tissue paper. While in use, the bulb of the glass
electrode and the diaphragm of the reference electrode should be immersed in
solution. For short-term storage the combined glass electrode should be
immersed in solution of 2 M
KCl. Buffer solutions of known pH are used for the pH
calibration. The pH values of some buffers are temperature dependent. For high
accuracy, calibration and measurements are to be performed at the same
temperature.
 |
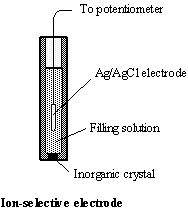
Ion-selective
electrodes are used for detection of specific ions in a mixture of
ions. The sensor element, ion-selective membrane, has a construction similar to
that of glass electrode. For calibration of ion-selective electrodes a standard
addition method is often employed.
 |
Silver
indicating electrodes are silver wires with 1-2 mm diameter. When used in
precipitation titration, the silver-salt precipitate should be occasionally
removed from the electrode surface (mechanically with fine grade emery paper,
or chemically immersing the electrode in NH3 solution). It is
simpler, however, to prevent the coating of the electrodes by addition of a
surfactant as polyvinyl alcohol (1 drop 0.3% PVA to every 5 ml of solution).
Mercury-coated
indicating electrodes are reported to be prepared by lightly amalgamating
a gold wire. The disadvantage in use of gold is that it is consumed with time
by the amalgam formation. Silver wire used instead of gold, however, can serve
many years. The preparation of mercury-coated silver electrode is done by the
instructor in a hood (mercury vapors are poisonous!). The silver wire (~1.5 mm
diameter) is (a) rubbed with emery paper, rinsed with distilled water and dried
with tissue; (b) dipped into mercury to form an amalgame;
(c) the mercury is gently spread on the wire with soft tissue. This electrode
may be used during several runs of titrations without any renewal. For renewal,
step (a) may be omitted.
Platinum
redox electrodes are used in redox potentiometric titrations.
In excess of oxidant oxide films are formed on the platinum electrodes. The
potential response of the electrode is distorted, and the film must be removed.
Efficient pretreatment is achieved by cathodically
polarizing the platinum electrode in 0.5 M H2SO4 at current
density of 0.5 mA/cm2 for 5 - 15 min. Platinum wire is recommended
to use as an auxiliary electrode.
Gold
redox electrodes are seldom used in potentiometric titrations. According to our recent
experience, the gold electrodes are better behaved than platinum electrodes in
view of rate of response and stability toward formation of oxides. These
features are of high importance in continuous mode of titration. A good example
is the use of gold electrodes in the titration of ascorbic acid with bromine in
continuous mode, where the response of the platinum electrode is
unsatisfactory.
Reference
electrodes. Calomel and silver/silver-chloride electrodes are commonly
used in potentiometric titration. In the case of
possible interferences of chlorides (as in determination of halides), a mercurous sulfate electrode may be used. In the following
series of experiments a home made Ag/AgCl/1 M KCl reference electrode is used. Its potential is -19
mV vs SCE, at 250C.
 |
Exp. 1. Compexometric
titration of Ca2+ and Mg2+ in drinking water
Chelating agents
A
chelating agent (ligand) is a multidendate
compexing molecule, forming usually a 1:1 complex (chelate) with a metal ion Mz+,
regardless of the value of the charge z of the metal ion.
Complexometric titration using chelating agent Y
(comprising n complexing groups) is far more
advantageous than titration with monodendate ligand L (of the same type), for two reasons:
(1) The chelate MY is more
stable than the MLn complex due to
thermodynamic considerations. The free energy change, 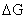 ,
,
is characterized by similar 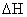 values, but very
different
values, but very
different 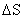 values, were
values, were 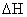 is the enthalpy and
is the enthalpy and 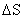 is the entropy. More
disorder is created by the dissociation of MLn
with the formation of n+1 species, compared to two species for the chelate.
is the entropy. More
disorder is created by the dissociation of MLn
with the formation of n+1 species, compared to two species for the chelate.
(2) Stepwise complexation
with monodendate ligands is
usually characterized with successive formation constants relatively close to
each other. Thus, there are several types of complexes in the vicinity of the
end point, compared to a chelate, where a single 1:1
complex is usually formed.
One of
the most widely used chelating agents is EDTA (ethylenediaminetetraacetic acid) with six complexing groups (two nitrogen and four carboxylic
groups). EDTA titrations have been applied to the determination of most
metal cations with the exception of the alkali metal
ions. Selectivity is obtained by controlling the pH. Ca2+ and Mg2+
have however close formation constants (Kf
= 5.0·1010 and 4.9·108 respectively), unsuitable for
separate detection. Titrating with EDTA, the total concentration of Ca2+
and Mg2+ is determined.
Read more at:
No comments:
Post a Comment