|
IRON AND STEEL
This page looks at the use of the Blast Furnace in the extraction of
iron from iron ore, and the conversion of the raw iron from the furnace
into various kinds of steel. Extracting iron from iron ore using a Blast Furnace Introduction The common ores of iron are both iron oxides, and these can be reduced to iron by heating them with carbon in the form of coke. Coke is produced by heating coal in the absence of air. Coke is cheap and provides both the reducing agent for the reaction and also the heat source - as you will see below. Iron ores The most commonly used iron ores are haematite (US: hematite), Fe2O3, and magnetite, Fe3O4. | |||||||||||||||||
|
Note: The two equations for the reduction of the ore on this page are for haematite. In the fairly unlikely event that you need the equations for magnetite, they aren't difficult to work out for yourself. | |||||||||||||||||
The Blast Furnace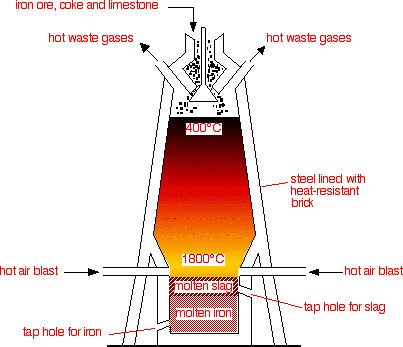 The air blown into the bottom of the furnace is heated using the hot waste gases from the top. Heat energy is valuable, and it is important not to waste any. The coke (essentially impure carbon) burns in the blast of hot air to form carbon dioxide - a strongly exothermic reaction. This reaction is the main source of heat in the furnace. The reduction of the ore At the high temperature at the bottom of the furnace, carbon dioxide reacts with carbon to produce carbon monoxide. It is the carbon monoxide which is the main reducing agent in the furnace. In the hotter parts of the furnace, the carbon itself also acts as a reducing agent. Notice that at these temperatures, the other product of the reaction is carbon monoxide, not carbon dioxide. The temperature of the furnace is hot enough to melt the iron which trickles down to the bottom where it can be tapped off. The function of the limestone Iron ore isn't pure iron oxide - it also contains an assortment of rocky material. This wouldn't melt at the temperature of the furnace, and would eventually clog it up. The limestone is added to convert this into slag which melts and runs to the bottom. The heat of the furnace decomposes the limestone to give calcium oxide. This is an endothermic reaction, absorbing heat from the furnace. It is therefore important not to add too much limestone because it would otherwise cool the furnace. Calcium oxide is a basic oxide and reacts with acidic oxides such as silicon dioxide present in the rock. Calcium oxide reacts with silicon dioxide to give calcium silicate. The calcium silicate melts and runs down through the furnace to form a layer on top of the molten iron. It can be tapped off from time to time as slag. Slag is used in road making and as "slag cement" - a final ground slag which can be used in cement, often mixed with Portland cement. Cast iron The molten iron from the bottom of the furnace can be used as cast iron. Cast iron is very runny when it is molten and doesn't shrink much when it solidifies. It is therefore ideal for making castings - hence its name. However, it is very impure, containing about 4% of carbon. This carbon makes it very hard, but also very brittle. If you hit it hard, it tends to shatter rather than bend or dent. Cast iron is used for things like manhole covers, guttering and drainpipes, cylinder blocks in car engines, Aga-type cookers, and very expensive and very heavy cookware. | |||||||||||||||||
|
Note: It is quite difficult to find examples of uses for cast iron, because it is nowadays often replaced by other materials. For example, although guttering and drainpipes were once made of cast iron, apart from special old buildings, it is now quite hard to find any which aren't made of plastic! | |||||||||||||||||
|
Steel
Most of the molten iron from a Blast Furnace is used to make one of a
number of types of steel. There isn't just one substance called steel -
they are a family of alloys of iron with carbon or various metals.
More about this later . . . Steel-making: the basic oxygen process Impurities in the iron from the Blast Furnace include carbon, sulphur, phosphorus and silicon. These have to be removed. Removal of sulphur Sulphur has to be removed first in a separate process. Magnesium powder is blown through the molten iron and the sulphur reacts with it to form magnesium sulphide. This forms a slag on top of the iron and can be removed. Removal of carbon etc The still impure molten iron is mixed with scrap iron (from recycling) and oxygen is blown on to the mixture. The oxygen reacts with the remaining impurities to form various oxides. The carbon forms carbon monoxide. Since this is a gas it removes itself from the iron! This carbon monoxide can be cleaned and used as a fuel gas. Elements like phosphorus and silicon react with the oxygen to form acidic oxides. These are removed using quicklime (calcium oxide) which is added to the furnace during the oxygen blow. They react to form compounds such as calcium silicate or calcium phosphate which form a slag on top of the iron. Types of iron and steel Cast iron has already been mentioned above. This section deals with the types of iron and steel which are produced as a result of the steel-making process. Wrought iron If all the carbon is removed from the iron to give high purity iron, it is known as wrought iron. Wrought iron is quite soft and easily worked and has little structural strength. It was once used to make decorative gates and railings, but these days mild steel is normally used instead. Mild steel Mild steel is iron containing up to about 0.25% of carbon. The presence of the carbon makes the steel stronger and harder than pure iron. The higher the percentage of carbon, the harder the steel becomes. Mild steel is used for lots of things - nails, wire, car bodies, ship building, girders and bridges amongst others. High carbon steel High carbon steel contains up to about 1.5% of carbon. The presence of the extra carbon makes it very hard, but it also makes it more brittle. High carbon steel is used for cutting tools and masonry nails (nails designed to be driven into concrete blocks or brickwork without bending). You have to be careful with high carbon steel because it tends to fracture rather than bend if you mistreat it. Special steels These are iron alloyed with other metals. For example:
| |||||||||||||||||
|
Note: This is deliberately brief because a lot of it is just common sense, and you will probably already have met it in detail in earlier chemistry courses, in geography, in general studies, or wherever. If you aren't sure about the various environmental problems like acid rain, global warming and the like, the very best site to find out about them is the US Environmental Protection Agency. | |||||||||||||||||
|
Environmental problems in mining and transporting the raw materials Think about:
Think about:
Think about:
| |||||||||||||||||
Thursday, 5 September 2013
IRON AND STEEL
Labels:
Inorganic Chemistry
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment