|
ATOMIC AND PHYSICAL PROPERTIES OF THE GROUP 7 ELEMENTS (THE HALOGENS)
This page explores the trends in some atomic and physical properties
of the Group 7 elements (the halogens) - fluorine, chlorine, bromine and
iodine. You will find separate sections below covering the trends in
atomic radius, electronegativity, electron affinity, melting and boiling
points, and solubility. There is also a section on the bond enthalpies
(strengths) of halogen-halogen bonds (for example, Cl-Cl) and of
hydrogen-halogen bonds (e.g. H-Cl) Even if you aren't currently interested in all these things, it would probably pay you to read the whole page. The same ideas tend to recur throughout the atomic properties, and you may find that earlier explanations help to you understand later ones. Trends in Atomic Radius | |||||||||
|
Note: You will find atomic radius covered in detail in another part of this site. If you choose to follow this link, use the BACK button on your browser to return quickly to this page. | |||||||||
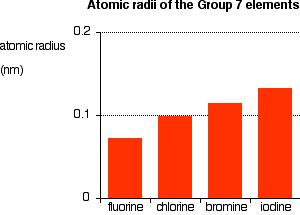 Explaining the increase in atomic radius The radius of an atom is governed by
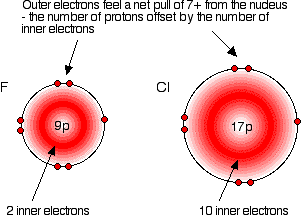 The only factor which is going to affect the size of the atom is therefore the number of layers of inner electrons which have to be fitted in around the atom. Obviously, the more layers of electrons you have, the more space they will take up - electrons repel each other. That means that the atoms are bound to get bigger as you go down the Group. Trends in Electronegativity Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is usually measured on the Pauling scale, on which the most electronegative element (fluorine) is given an electronegativity of 4.0. | |||||||||
|
Note: You will find electronegativity covered in detail in another part of this site. If you choose to follow this link, use the BACK button on your browser to return quickly to this page. | |||||||||
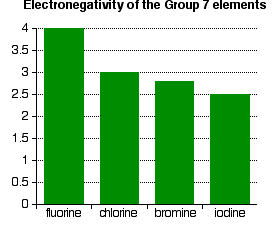 Explaining the decrease in electronegativity This is easily shown using simple dots-and-crosses diagrams for hydrogen fluoride and hydrogen chloride. 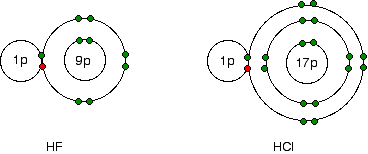 The larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. Summarising the trend down the Group As the halogen atoms get bigger, any bonding pair gets further and further away from the halogen nucleus, and so is less strongly attracted towards it. In other words, as you go down the Group, the elements become less electronegative. Trends in First Electron Affinity Defining first electron affinity The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. This is more easily seen in symbol terms. It is the energy released (per mole of X) when this change happens. First electron affinities have negative values. For example, the first electron affinity of chlorine is -349 kJ mol-1. By convention, the negative sign shows a release of energy. The first electron affinities of the Group 7 elements | |||||||||
|
Note: You will find electron affinity covered in detail in another part of this site. The current page duplicates much of that material, but you might like to read it again in different words. If you choose to follow this link, use the BACK button on your browser to return quickly to this page. | |||||||||
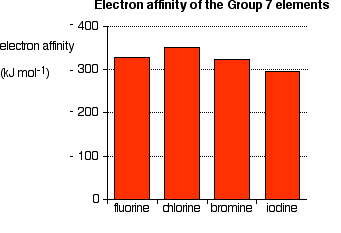 The electron affinity is a measure of the attraction between the incoming electron and the nucleus. The higher the attraction, the higher the electron affinity. 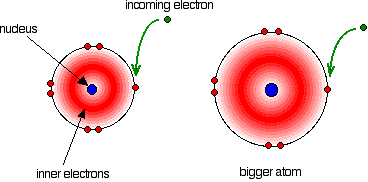 As the atom gets bigger, the incoming electron is further from the nucleus and so feels less attraction. The electron affinity therefore falls as you go down the Group. But what about fluorine? That is a very small atom, with the incoming electron quite close to the nucleus. Why isn't its electron affinity bigger than chlorine's? There is another effect operating. When the new electron comes into the atom, it is entering a region of space already very negatively charged because of the existing electrons. There is bound to be some repulsion, offsetting some of the attraction from the nucleus. In the case of fluorine, because the atom is very small, the existing electron density is very high. That means that the extra repulsion is particularly great and lessens the attraction from the nucleus enough to lower the electron affinity below that of chlorine. Trends in Melting Point and Boiling Point 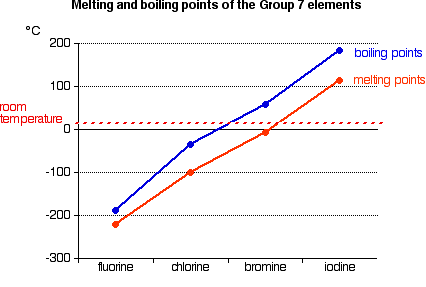 If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature, bromine is a liquid and iodine a solid. Nothing very surprising there! Explaining the trends in melting point and boiling point All of the halogens exist as diatomic molecules - F2, Cl2, and so on. The intermolecular attractions between one molecule and its neighbours are van der Waals dispersion forces. | |||||||||
|
Note: If you aren't sure about van der Waals dispersion forces, you will find them covered in detail in another part of this site. You won't understand the next bit unless you are happy about dispersion forces and how they vary with the size of the molecule. Use the BACK button on your browser to return quickly to this page. | |||||||||
|
As the molecules get bigger there are obviously more electrons which
can move around and set up the temporary dipoles which create these
attractions. The stronger intermolecular attractions as the molecules get bigger means that you have to supply more heat energy to turn them into either a liquid or a gas - and so their melting and boiling points rise. Solubilities Solubility in water Fluorine reacts violently with water to give hydrogen fluoride gas (or a solution of hydrofluoric acid) and a mixture of oxygen and ozone. So thinking about its solubility is pointless. Chlorine, bromine and iodine all dissolve in water to some extent, but there is no pattern in this. The following table shows the solubility of the three elements in water at 25°C.
| |||||||||
|
Note: These figures come from page 476 of Advanced Inorganic Chemistry (third edition) by Cotton and Wilkinson. | |||||||||
|
Chlorine solution in water is pale green. Bromine solution in water
is anything from yellow to dark orange-red depending on how concentrated
it is. Iodine solution in water is very pale brown. Chlorine reacts with water to some extent to give a mixture of hydrochloric acid and chloric(I) acid - also known as hypochlorous acid. The reaction is reversible, and at any one time only about a third of the chlorine molecules have actually reacted. You will sometimes find the chloric(I) acid written as HOCl. That represents the way the atoms are actually joined together. Bromine and iodine do something similar, but to a much lesser extent. In both cases, about 99.5% of the halogen remains as unreacted molecules. The solubility of iodine in potassium iodide solution Although iodine is only faintly soluble in water, it does dissolve freely in potassium iodide solution to give a dark red-brown solution. There is a reversible reaction between iodine molecules and iodide ions to give I3- ions. These are responsible for the colour. In the lab, iodine is often produced by oxidation of a solution containing iodide ions, so this colour is actually quite familiar. As long as there are any excess iodide ions present, the iodine will react with them to make the I3- ions. Once the iodide ions have all reacted, the iodine is precipitated as a dark grey solid, because there isn't anything left for it to react with to keep it in solution. Solubility in hexane The halogens are much more soluble in organic solvents like hexane than they are in water. Both hexane and the halogens are non-polar molecules attracted to each other by van der Waals dispersion forces. That means that the attractions broken (between hexane molecules and between halogen molecules) are similar to the new attractions made when the two substances mix. The colours of the solutions formed are much what you would expect. Solutions of iodine in organic solvents tend to be pinky-purple colour. Bond enthalpies (bond energies or bond strengths) Bond enthalpy is the heat needed to break one mole of a covalent bond to produce individual atoms, starting from the original substance in the gas state, and ending with gaseous atoms. So for chlorine, Cl2(g), it is the heat energy needed to carry out this change per mole of bond: For bromine, the reaction is still from gaseous bromine molecules to separate gaseous atoms. 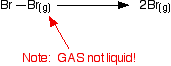 Bond enthalpy in the halogens, X2(g) A covalent bond works because the bonding pair is attracted to both the nuclei at either side of it. It is that attraction which holds the molecule together. The size of the attraction will depend, amongst other things, on the distance from the bonding pair to the two nuclei. 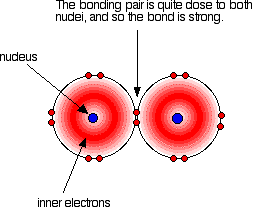 As the atoms get bigger, the bonding pair gets further from the nuclei and so you would expect the strength of the bond to fall. 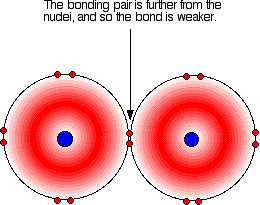 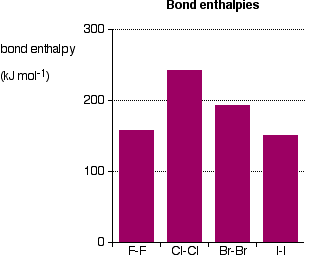 Because fluorine atoms are so small, you might expect a very strong bond - in fact, it is remarkably weak. There must be another factor at work as well. As well as the bonding pair of electrons between the two atoms, each atom has 3 non-bonding pairs of electrons in the outer level - lone pairs. Where the bond gets very short (as in F-F), the lone pairs on the two atoms get close enough together to set up a significant amount of repulsion. 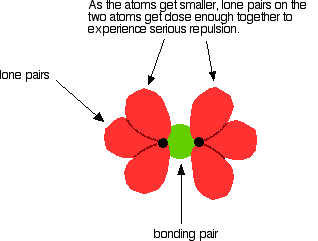 Bond enthalpies in the hydrogen halides, HX(g) Where the halogen atom is attached to a hydrogen atom, this effect doesn't happen. There are no lone pairs on a hydrogen atom! 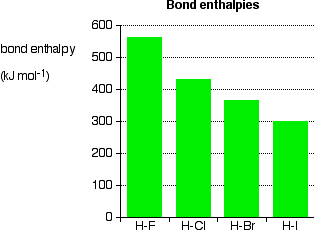 This is important in the thermal stability of the hydrogen halides - how easily they are broken up into hydrogen and the halogen on heating. Hydrogen fluoride and hydrogen chloride are very stable to heat. They don't split up into hydrogen and fluorine or chlorine again if heated to any normal lab temperature. Hydrogen bromide splits slightly into hydrogen and bromine on heating, and hydrogen iodide splits to an even greater extent. As the bonds get weaker, they are more easily broken. | |||||||||
|
Note: Breaking the hydrogen-halogen bond is only one of the steps in the overall reaction, of course. You don't end up with hydrogen atoms and halogen atoms - you get diatomic molecules, H2 and X2. If you have done some energetics calculations, it would be a useful exercise to use bond enthalpies and atomisation enthalpies to calculate the overall enthalpy changes for the decomposition of the hydrogen halides. (Remember that bond enthalpies only apply to substances in the gas state, and bromine and iodine would end up as liquid and solid respectively. Using atomisation enthalpies for the halogens avoids this problem. If you don't understand what I am talking about, you don't yet have enough knowledge to be able to do this.) | |||||||||
Thursday, 5 September 2013
ATOMIC AND PHYSICAL PROPERTIES OF THE GROUP 7 ELEMENTS (THE HALOGENS)
Labels:
Inorganic Chemistry
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment