Structures
of Metals
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
What is a metal ? Metal Properties Metal processing |
Metals account for about two thirds of all the elements and about 24%
of the mass of the planet. They are all around us in such forms as steel
structures, copper wires, aluminum foil, and gold jewelry. Metals are
widely used because of their properties: strength,
ductility, high melting point, thermal and electrical conductivity, and toughness.
These properties also offer clues as to the structure of metals. As with all elements, metals are composed of atoms. The strength of metals suggests that these atoms are held together by strong bonds. These bonds must also allow atoms to move; otherwise how could metals be hammered into sheets or drawn into wires? A reasonable model would be one in which atoms are held together by strong, but delocalized, bonds. Bonding Such bonds could be formed between metal atoms that have low electronegativities and do not attract their valence electrons strongly. This would allow the outermost electrons to be shared by all the surrounding atoms, resulting in positive ions (cations) surrounded by a sea of electrons (sometimes referred to as an electron cloud). 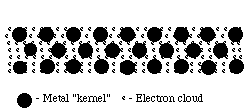 Because these valence electrons are shared by all the atoms, they are not considered to be associated with any one atom. This is very different from ionic or covalent bonds, where electrons are held by one or two atoms. The metallic bond is therefore strong and uniform. Since electrons are attracted to many atoms, they have considerable mobility that allows for the good heat and electrical conductivity seen in metals. Above their melting point, metals are liquids, and their atoms are randomly arranged and relatively free to move. However, when cooled below their melting point, metals rearrange to form ordered, crystalline structures. 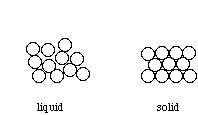 To form the strongest metallic bonds, metals are packed together as closely as possible. Several packing arrangements are possible. Instead of atoms, imagine marbles that need to be packed in a box. The marbles would be placed on the bottom of the box in neat orderly rows and then a second layer begun. The second layer of marbles cannot be placed directly on top of the other marbles and so the rows of marbles in this layer move into the spaces between marbles in the first layer. The first layer of marbles can be designated as A and the second layer as B giving the two layers a designation of AB.
Packing marbles in the third layer requires a decision. Again rows of atoms will nest in the hollows between atoms in the second layer but two possibilities exist. If the rows of marbles are packed so they are directly over the first layer (A) then the arrangement could be described as ABA. Such a packing arrangement with alternating layers would be designated as ABABAB. This ABAB arrangement is called hexagonal close packing (HCP). If the rows of atoms are packed in this third layer so that they do not lie over atoms in either the A or B layer, then the third layer is called C. This packing sequence would be designated ABCABC, and is also known as face-centered cubic (FCC). Both arrangements give the closest possible packing of spheres leaving only about a fourth of the available space empty. The smallest repeating array of atoms in a crystal is called a unit cell. A third common packing arrangement in metals, the body-centered cubic (BCC) unit cell has atoms at each of the eight corners of a cube plus one atom in the center of the cube. Because each of the corner atoms is the corner of another cube, the corner atoms in each unit cell will be shared among eight unit cells. The BCC unit cell consists of a net total of two atoms, the one in the center and eight eighths from the corners. In the FCC arrangement, again there are eight atoms at corners of the unit cell and one atom centered in each of the faces. The atom in the face is shared with the adjacent cell. FCC unit cells consist of four atoms, eight eighths at the corners and six halves in the faces. Table 1 shows the stable room temperature crystal structures for several elemental metals.
Unit cell structures determine some of the properties of metals. For example, FCC structures are more likely to be ductile than BCC, (body centered cubic) or HCP (hexagonal close packed). Figure 4 shows the FCC and BCC unit cells. (See Crystal Structure Activity)
As atoms of melted metal begin to pack together to form a crystal lattice at the freezing point, groups of these atoms form tiny crystals. These tiny crystals increase in size by the progressive addition of atoms. The resulting solid is not one crystal but actually many smaller crystals, called grains. These grains grow until they impinge upon adjacent growing crystals. The interface formed between them is called a grain boundary. Grains are sometimes large enough to be visible under an ordinary light microscope or even to the unaided eye. The spangles that are seen on newly galvanized metals are grains. (See A Particle Model of Metals Activity) Figure 5 shows a typical view of a metal surface with many grains, or crystals.  Crystal Defects: Metallic crystals are not perfect. Sometimes there are empty spaces called vacancies, where an atom is missing. Another common defect in metals are dislocations, which are lines of defective bonding. Figure 6 shows one type of dislocation. 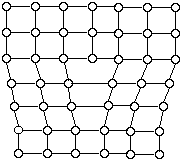 These and other imperfections, as well as the existence of grains and grain boundaries, determine many of the mechanical properties of metals. When a stress is applied to a metal, dislocations are generated and move, allowing the metal to deform. | |||||||||||||||||||||||||||||||||||||||||||||||||||||
Wednesday, 17 July 2013
Structures of Metals
Subscribe to:
Post Comments (Atom)





No comments:
Post a Comment