Naming Organic Compounds
As organic chemistry grew and developed, many compounds were given trivial names, which are now commonly used and recognized. Some examples are:
| Name | Methane | Butane | Acetone | Toluene | Acetylene | Ethyl Alcohol |
|---|---|---|---|---|---|---|
| Formula | CH4 | C4H10 | CH3COCH3 | CH3C6H5 | C2H2 | C2H5OH |
The IUPAC Systematic Approach to Nomenclature
The IUPAC nomenclature system is a set of logical rules devised and used by organic chemists to circumvent problems caused by arbitrary nomenclature. Knowing these rules and given a structural formula, one should be able to write a unique name for every distinct compound. Likewise, given a IUPAC name, one should be able to write a structural formula. In general, an IUPAC name will have three essential features:
• A suffix or other element(s) designating functional groups that may be present in the compound.
• Names of substituent groups, other than hydrogen, that complete the molecular structure.
An excellent presentation of organic nomenclature is provided on a Nomenclature Page. created by Dave Woodcock.
A full presentation of the IUPAC Rules is also available.
Alkanes |
|---|
Alkanes
Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes, depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework on which functional groups are located in other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. The alkanes and cycloalkanes are also members of a larger class of compounds referred to as aliphatic. Simply put, aliphatic compounds are compounds that do not incorporate any aromatic rings in their molecular structure.The following table lists the IUPAC names assigned to simple continuous-chain alkanes from C-1 to C-10. A common "ane" suffix identifies these compounds as alkanes. Longer chain alkanes are well known, and their names may be found in many reference and text books. The names methane through decane should be memorized, since they constitute the root of many IUPAC names. Fortunately, common numerical prefixes are used in naming chains of five or more carbon atoms.
| Name | Molecular Formula | Structural Formula | Isomers | Name | Molecular Formula | Structural Formula | Isomers | |
|---|---|---|---|---|---|---|---|---|
| methane | CH4 | CH4 | 1 | hexane | C6H14 | CH3(CH2)4CH3 | 5 | |
| ethane | C2H6 | CH3CH3 | 1 | heptane | C7H16 | CH3(CH2)5CH3 | 9 | |
| propane | C3H8 | CH3CH2CH3 | 1 | octane | C8H18 | CH3(CH2)6CH3 | 18 | |
| butane | C4H10 | CH3CH2CH2CH3 | 2 | nonane | C9H20 | CH3(CH2)7CH3 | 35 | |
| pentane | C5H12 | CH3(CH2)3CH3 | 3 | decane | C10H22 | CH3(CH2)8CH3 | 75 |
(ii) A uniform variation of this kind in a series of compounds is called homologous.
(iii) These formulas all fit the CnH2n+2 rule. This is also the highest possible H/C ratio for a stable hydrocarbon.
(iv) Since the H/C ratio in these compounds is at a maximum, we call them saturated (with hydrogen).
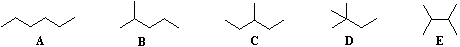
The IUPAC system requires first that we have names for simple unbranched chains, as noted above, and second that we have names for simple alkyl groups that may be attached to the chains. Examples of some common alkyl groups are given in the following table. Note that the "ane" suffix is replaced by "yl" in naming groups. The symbol R is used to designate a generic (unspecified) alkyl group.
| Group | CH3– | C2H5– | CH3CH2CH2– | (CH3)2CH– | CH3CH2CH2CH2– | (CH3)2CHCH2– | CH3CH2CH(CH3)– | (CH3)3C– | R– |
| Name | Methyl | Ethyl | Propyl | Isopropyl | Butyl | Isobutyl | sec-Butyl | tert-Butyl | Alkyl |
IUPAC Rules for Alkane Nomenclature2. Identify and name groups attached to this chain. 3. Number the chain consecutively, starting at the end nearest a substituent group. 4. Designate the location of each substituent group by an appropriate number and name. 5. Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. |
For additional examples of how these rules are used in naming branched alkanes, and for some sub-rules of nomenclature .
Cycloalkanes |
|---|
Cycloalkanes
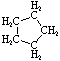
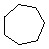
Cycloalkanes have one or more rings of carbon atoms. The simplest examples of this class consist of a single, unsubstituted carbon ring, and these form a homologous series similar to the unbranched alkanes. The IUPAC names of the first five members of this series are given in the following table. The last (yellow shaded) column gives the general formula for a cycloalkane of any size. If a simple unbranched alkane is converted to a cycloalkane two hydrogen atoms, one from each end of the chain, must be lost. Hence the general formula for a cycloalkane composed of n carbons is CnH2n. Although a cycloalkane has two fewer hydrogens than the equivalent alkane, each carbon is bonded to four other atoms so such compounds are still considered to be saturated with hydrogen.| Name | Cyclopropane | Cyclobutane | Cyclopentane | Cyclohexane | Cycloheptane | Cycloalkane |
|---|---|---|---|---|---|---|
| Molecular Formula |
C3H6 | C4H8 | C5H10 | C6H12 | C7H14 | CnH2n |
| Structural Formula |
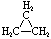 |
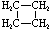 |
 |
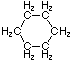 |
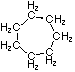 |
(CH2)n |
| Line Formula |
 |
 |
 |
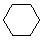 |
 |
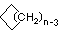 |
Over eight years of successful operation in the pharmaceutical industry has enabled us to possess a full-bodied array of building blocks in numerous pack sizes. 7-O-ethyl-morroniside
ReplyDelete