|
THE MASS SPECTRA OF ELEMENTS
This page looks at the information you can get from the mass spectrum
of an element. It shows how you can find out the masses and relative
abundances of the various isotopes of the element and use that
information to calculate the relative atomic mass of the element. It also looks at the problems thrown up by elements with diatomic molecules - like chlorine, Cl2. The mass spectrum of monatomic elements Monatomic elements include all those except for things like chlorine, Cl2, with molecules containing more than one atom. The mass spectrum for boron | |||||||||||||||||||||||
|
Note: If you need to know how this diagram is obtained, you should read the page describing how a mass spectrometer works. | |||||||||||||||||||||||
|
The number of isotopes The two peaks in the mass spectrum shows that there are 2 isotopes of boron - with relative isotopic masses of 10 and 11 on the 12C scale. | |||||||||||||||||||||||
|
Notes: Isotopes are atoms of the same element (and so with the same number of protons), but with different masses due to having different numbers of neutrons. We are assuming (and shall do all through this page) that all the ions recorded have a charge of 1+. That means that the mass/charge ratio (m/z) gives you the mass of the isotope directly. The carbon-12 scale is a scale on which the mass of the 12C isotope weighs exactly 12 units. | |||||||||||||||||||||||
|
The abundance of the isotopes The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. The tallest peak is often given an arbitrary height of 100 - but you may find all sorts of other scales used. It doesn't matter in the least. You can find the relative abundances by measuring the lines on the stick diagram. In this case, the two isotopes (with their relative abundances) are:
The relative atomic mass (RAM) of an element is given the symbol Ar and is defined as:
Suppose you had 123 typical atoms of boron. 23 of these would be 10B and 100 would be 11B. The total mass of these would be (23 x 10) + (100 x 11) = 1330 The average mass of these 123 atoms would be 1330 / 123 = 10.8 (to 3 significant figures). 10.8 is the relative atomic mass of boron. Notice the effect of the "weighted" average. A simple average of 10 and 11 is, of course, 10.5. Our answer of 10.8 allows for the fact that there are a lot more of the heavier isotope of boron - and so the "weighted" average ought to be closer to that. The mass spectrum for zirconium The 5 peaks in the mass spectrum shows that there are 5 isotopes of zirconium - with relative isotopic masses of 90, 91, 92, 94 and 96 on the 12C scale. The abundance of the isotopes This time, the relative abundances are given as percentages. Again you can find these relative abundances by measuring the lines on the stick diagram. In this case, the 5 isotopes (with their relative percentage abundances) are:
| |||||||||||||||||||||||
|
Note: You almost certainly wouldn't be able to measure these peaks to this degree of accuracy, but your examiners may well give you the data in number form anyway. We'll do the sum with the more accurate figures. | |||||||||||||||||||||||
|
Working out the relative atomic mass Suppose you had 100 typical atoms of zirconium. 51.5 of these would be 90Zr, 11.2 would be 91Zr and so on. | |||||||||||||||||||||||
|
Note: If you object to the idea of having 51.5 atoms or 11.2 atoms and so on, just assume you've got 1000 atoms instead of 100. That way you will have 515 atoms, 112 atoms, etc. Most people don't get in a sweat over this, and just use the numbers as they are! | |||||||||||||||||||||||
|
The total mass of these 100 typical atoms would be (51.5 x 90) + (11.2 x 91) + (17.1 x 92) + (17.4 x 94) + (2.8 x 96) = 9131.8 The average mass of these 100 atoms would be 9131.8 / 100 = 91.3 (to 3 significant figures). 91.3 is the relative atomic mass of zirconium. | |||||||||||||||||||||||
|
Note: If you want further examples of calculating relative atomic masses from mass spectra, you might like to refer to my book, Calculations in A level Chemistry. | |||||||||||||||||||||||
|
The mass spectrum of chlorine
Chlorine is taken as typical of elements with more than one atom per
molecule. We'll look at its mass spectrum to show the sort of problems
involved. Chlorine has two isotopes, 35Cl and 37Cl, in the approximate ratio of 3 atoms of 35Cl to 1 atom of 37Cl. You might suppose that the mass spectrum would look like this: The problem is that chlorine consists of molecules, not individual atoms. When chlorine is passed into the ionisation chamber, an electron is knocked off the molecule to give a molecular ion, Cl2+. These ions won't be particularly stable, and some will fall apart to give a chlorine atom and a Cl+ ion. The term for this is fragmentation. If the Cl atom formed isn't then ionised in the ionisation chamber, it simply gets lost in the machine - neither accelerated nor deflected. The Cl+ ions will pass through the machine and will give lines at 35 and 37, depending on the isotope and you would get exactly the pattern in the last diagram. The problem is that you will also record lines for the unfragmented Cl2+ ions. Think about the possible combinations of chlorine-35 and chlorine-37 atoms in a Cl2+ ion. Both atoms could be 35Cl, both atoms could be 37Cl, or you could have one of each sort. That would give you total masses of the Cl2+ ion of:
The relative heights of the 70, 72 and 74 lines are in the ratio 9:6:1. If you know the right bit of maths, it's very easy to show this. If not, don't worry. Just remember that the ratio is 9:6:1. What you can't do is make any predictions about the relative heights of the lines at 35/37 compared with those at 70/72/74. That depends on what proportion of the molecular ions break up into fragments. That's why you've got the chlorine mass spectrum in two separate bits so far. You must realise that the vertical scale in the diagrams of the two parts of the spectrum isn't the same. The overall mass spectrum looks like this: 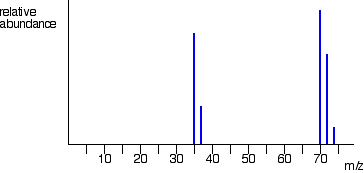 | |||||||||||||||||||||||
|
Note: This is based on information from the NIST Chemistry WebBook. NIST is the US National Institute of Standards and Technology. | |||||||||||||||||||||||
Saturday, 13 July 2013
THE MASS SPECTRA OF ELEMENTS
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment