Organic chemistry is the chemistry of carbon compounds. All organic
compounds contain carbon; however, there are some compounds of carbon that
are not classified as organic. For example, salts such as carbonates
(e.g., Na
2
CO
3
, CaCO
3
) and cyanides (e.g., NaCN, KCN) are usually designated as inorganic.
Perhaps a more useful description might be: Organic compounds are
compounds of carbon that usually contain hydrogen and that may also
contain other elements such as oxygen, nitrogen, sulfur, phosphorus, or
halogen
(F, Cl, Br, or I). In any case, there are very few carbon compounds that
are not organic, while there are millions that are.
During the Middle Ages dry distillation of wood yielded mixtures of methyl alcohol, acetone, and acetic acid. Alchemists isolated cholesterol from gallstones, morphine from opium, and drugs such as quinine, strychnine, and brucine from various plants. Two hundred years ago chemists such as Antoine Lavoisier determined the elemental composition of many of these substances and noted that they all contained carbon and hydrogen, and that many also contained oxygen and nitrogen. It also appeared that there were two classes of materials: the mineral type (generally hard, high-melting, and noncombustible), and the organic type (often soft, liquid or low melting solids, and frequently easily combustible materials). Most organic chemicals could be burned to produce carbon dioxide; and any hydrogen present was converted to water (H 2 O). Because organic compounds had for centuries been isolated only from plants and animals, it was commonly believed that some "vital force" in living things was necessary to produce them. This belief persisted until 1828, when Friedrich Wöhler was able to make urea, a chemical found in the urine of animals, from the inorganic salt ammonium cyanate.
Since that time organic chemistry has grown into a vast and ever expanding field that encompasses millions of chemical compounds.
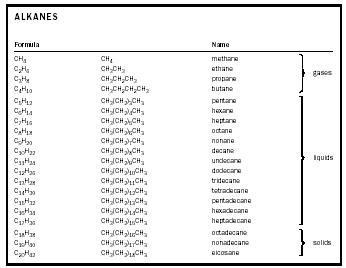
have ring structures. Since a 4-carbon chain of the alkane series is
called
butane,
a ring of 4 carbon atoms is called
cyclobutane.
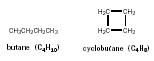 Simple hydrocarbons that contain one or more double bonds are called
alkenes.
They are named like alkanes, but their names end in "
–ene." The simplest alkene has two carbon atoms and is
called
ethene.
A 3-carbon chain that has a double bond is called
propene.
Simple hydrocarbons that contain one or more double bonds are called
alkenes.
They are named like alkanes, but their names end in "
–ene." The simplest alkene has two carbon atoms and is
called
ethene.
A 3-carbon chain that has a double bond is called
propene.
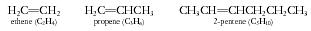 A 5-carbon hydrocarbon chain with a double bond is called
pentene,
and if the double bond links the second and third carbons, it is 2-
pentene.
Like cycloalkanes, alkenes have the general formula C
n
H
2n
. Alkenes having ring structures are called
cycloalkenes.
A 5-carbon ring with a double bond is called
cyclopentene.
A 5-carbon hydrocarbon chain with a double bond is called
pentene,
and if the double bond links the second and third carbons, it is 2-
pentene.
Like cycloalkanes, alkenes have the general formula C
n
H
2n
. Alkenes having ring structures are called
cycloalkenes.
A 5-carbon ring with a double bond is called
cyclopentene.
Hydrocarbons that contain one or more triple bonds are called alkynes, and is the name ending is "–yne." A 2-carbon alkyne is therefore named ethyne. (However, the compound is often referred to by its common name, which is acetylene. )
Compounds that contain double or triple bonds are said to be "unsaturated"—because they are not "saturated" with hydrogen atoms. Unsaturated compounds are reactive materials that readily add hydrogen when heated over a catalyst such as nickel. The reverse reaction also occurs. Heating ethane with steam is an important commercial process for making ethene (or ethylene). This is an important commercial process called "steam cracking."
When a 6-carbon ring contains 2 double bonds, it is called cyclohexadiene, but when it has 3 double bonds, it is not called cyclohexatriene; this is because a 6-carbon ring with three double bonds takes on a special kind of stability. The double bonds become completely conjugated and no longer behave as double bonds. The ring, known as a "benzene ring," is said to be aromatic.
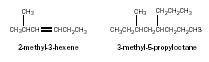
The removal of a hydrogen atom from a hydrocarbon molecule leaves an alkyl group that readily attaches to a functional group, or forms a branch on a hydrocarbon chain. The groups are named after the corresponding hydrocarbons. For example, CH 3 – is named methyl; CH 3 CH 2 –, ethyl; CH 2 = CH–, ethenyl; CH 3 CH 2 CH 2 –, propyl; and so on. A benzene ring from which a hydrogen atom has been removed is often referred to as a phenyl. The branched molecules shown here would be given names as follows
 Theoretically there is no limit to the length of hydrocarbon chains. Very
large hydrocarbon molecules (polymers) have been made containing as many
as 100,000 carbon atoms. However, such molecules are hard to make and very
difficult to melt and to shape into useful products.
Theoretically there is no limit to the length of hydrocarbon chains. Very
large hydrocarbon molecules (polymers) have been made containing as many
as 100,000 carbon atoms. However, such molecules are hard to make and very
difficult to melt and to shape into useful products.
Hydrocarbons are obtained primarily from fossil fuels—especially petroleum and natural gas. Natural gas is a mixture that is largely methane mixed with varying amounts of ethane and other light hydrocarbons, while petroleum is a complex mixture of many different hydrocarbons. Coal, the other fossil fuel, is a much more complicated material from which many kinds of organic compounds, some of them hydrocarbons, can be obtained.
 The linear molecule (1) is called butane, or
normal
butane (
n
-butane), whereas the branched molecule (2) is methylpropane (rather than
2-methylpropane, as the methyl group has to be in a 2-position). If the
methyl group of (2) were attached to a terminal carbon, the resultant
molecule would be the same as (1). Methylpropane (2) is also called
iso
butane.
The linear molecule (1) is called butane, or
normal
butane (
n
-butane), whereas the branched molecule (2) is methylpropane (rather than
2-methylpropane, as the methyl group has to be in a 2-position). If the
methyl group of (2) were attached to a terminal carbon, the resultant
molecule would be the same as (1). Methylpropane (2) is also called
iso
butane.
For molecules other than hydrocarbons, still other kinds of isomers are possible. The simple formula C 2 H 6 O can represent ethyl alcohol or dimethyl ether; and C 3 H 6 O could stand for an alcohol, an ether, an aldehyde , or a ketone (among other things). The larger a molecule is, and the greater the variety of atoms and functional groups it contains, the more numerous its isomers.
There is still another kind of isomerism that stems from the existence of "right-" and "left-handed" molecules. It is sometimes referred to as optical isomerism because the molecules that make up a pair of these isomers usually differ only in the way they rotate plane polarized light.
To bring some order to the naming process an international meeting was held in 1892 at Geneva, Switzerland. The group later became known as the International Union of Pure and Applied Chemistry (IUPAC). Its objective was to establish a naming process that would provide each compound with a unique and systematic name. An initial set of rules was adopted at that first meeting in Geneva, and IUPAC has continued that work. Its systematic naming rules are used by organic chemists all over the world. The names of the alkanes form the basis for the system, with functional groups usually being indicated with appropriate suffixes. Some examples are given in Table 2.
of solvent. Of course, not all organic reactions are highly successful.
One reaction might be a very simple one giving essentially 100 percent of
the desired product; but another might be a complex multistep process
yielding less than 5 percent overall of the wanted product.
Organic reactions can often give remarkable control as to what products should be formed. Adding water to propene for example, produces 2-propanol in the presence of acid, but it yields 1-propanol if treated first with B 2 H 6 and then H 2 O 2 in the presence of base.
Column chromatography, gas chromatography, and liquid chromatography are all important methods for separating mixtures of organic compounds. Spectroscopic tools include ultraviolet (UV), infrared (IR), nuclear magnetic resonance (NMR), and mass spectroscopy (MS), each capable of providing a different kind of information about an organic compound. Although it is limited to substances that can be prepared as pure crystals, x-ray crystallography is probably the ultimate tool for determining molecular structure.
History
Prehistoric civilizations obtained many useful chemicals from plants and animals. They were familiar with sugar, which they learned to ferment to make wine. Then they found that the wine could turn into vinegar. Ancient Egyptians used blue dye made from the indigo in madder root, and a royal purple dye extracted from a rare kind of mollusk. Soap was made by heating animal fat with base from wood ashes.During the Middle Ages dry distillation of wood yielded mixtures of methyl alcohol, acetone, and acetic acid. Alchemists isolated cholesterol from gallstones, morphine from opium, and drugs such as quinine, strychnine, and brucine from various plants. Two hundred years ago chemists such as Antoine Lavoisier determined the elemental composition of many of these substances and noted that they all contained carbon and hydrogen, and that many also contained oxygen and nitrogen. It also appeared that there were two classes of materials: the mineral type (generally hard, high-melting, and noncombustible), and the organic type (often soft, liquid or low melting solids, and frequently easily combustible materials). Most organic chemicals could be burned to produce carbon dioxide; and any hydrogen present was converted to water (H 2 O). Because organic compounds had for centuries been isolated only from plants and animals, it was commonly believed that some "vital force" in living things was necessary to produce them. This belief persisted until 1828, when Friedrich Wöhler was able to make urea, a chemical found in the urine of animals, from the inorganic salt ammonium cyanate.
Since that time organic chemistry has grown into a vast and ever expanding field that encompasses millions of chemical compounds.
Scope of Organic Chemistry
The field of organic chemistry includes more than twenty million compounds for which properties have been determined and recorded in the literature. Many hundreds of new compounds are added every day. Much more than half of the world's chemists are organic chemists. Some new organic compounds are simply isolated from plants or animals; some are made by modifying naturally occurring chemicals; but most new organic compounds are actually synthesized in the laboratory from other (usually smaller) organic molecules. Over the years organic chemists have developed a broad array of reactions that allow them to make all kinds of complex products from simpler starting materials.Singular Attributes of Carbon
When one considers the millions of chemical compounds that are known and notes that more than 95 percent of them are compounds of carbon, one realizes that carbon is unique. Why are there so many carbon compounds? It turns out that atoms of carbon are quite remarkable in a number of ways.Carbon atoms form very strong bonds with other carbon atoms. The bonds are so strong that carbon can form long chains, some containing thousands of carbon atoms. (Carbon is the only element that can do this.)
A carbon atom forms four bonds, therefore carbon not only can form long chains, but it also forms chains that have branches. It is a major reason why carbon compounds exhibit so much isomerism. The simple compound decane (C 10 H 22 ), for example, has 75 different isomers .
Carbon atoms can be bonded by double or triple bonds as well as single bonds. This multiple bonding is much more prevalent with carbon than with any other element.
Carbon atoms can form rings of various sizes. The rings may be saturated or unsaturated. The unsaturated 6-membered ring known as the benzene ring is the basis for an entire subfield of "aromatic" organic chemistry.
Carbon atoms form strong bonds not only with other carbon atoms but also with atoms of other elements. In addition to hydrogen, many carbon compounds also contain oxygen. Nitrogen, sulfur, phosphorus, and the halogens also frequently occur in carbon compounds.
Various kinds of functional groups occur widely among carbon compounds, and many different kinds of isomers are possible.
Hydrocarbons
Compounds of carbon and hydrogen only are called hydrocarbons. These are the simplest compounds of organic chemistry. The most basic group of hydrocarbons are the alkanes, which contain only single bonds. The simplest member of the alkane series is methane, CH 4 , the main component of natural gas. The names of some alkanes are listed in Table 1. Alkanes sometimes
Table 1.
| ALKANES | |||
| Formula | Name | ||
| CH 4 | CH 4 | methane | gases |
| C 2 H 6 | CH 3 CH 3 | ethane | |
| C 3 H 8 | CH 3 CH 2 CH 3 | propane | |
| C 4 H 10 | CH 3 CH 2 CH 2 CH 3 | butane | |
| C 5 H 12 | CH 3 (CH 2 ) 3 CH 3 | pentane | liquids |
| C 6 H 14 | CH 3 (CH 2 ) 4 CH 3 | hexane | |
| C 7 H 16 | CH 3 (CH 2 ) 5 CH 3 | heptane | |
| C 8 H 18 | CH 3 (CH 2 ) 6 CH 3 | octane | |
| C 9 H 20 | CH 3 (CH 2 ) 7 CH 3 | nonane | |
| C 10 H 22 | CH 3 (CH 2 ) 8 CH 3 | decane | |
| C 11 H 24 | CH 3 (CH 2 ) 9 CH 3 | undecane | |
| C 12 H 26 | CH 3 (CH 2 ) 10 CH 3 | dodecane | |
| C 13 H 28 | CH 3 (CH 2 ) 11 CH 3 | tridecane | |
| C 14 H 30 | CH 3 (CH 2 ) 12 CH 3 | tetradecane | |
| C 15 H 32 | CH 3 (CH 2 ) 13 CH 3 | pentadecane | |
| C 16 H 34 | CH 3 (CH 2 ) 14 CH 3 | hexadecane | |
| C 17 H 36 | CH 3 (CH 2 ) 15 CH 3 | heptadecane | |
| C 18 H 38 | CH 3 (CH 2 ) 16 CH 3 | octadecane | solids |
| C 19 H 40 | CH 3 (CH 2 ) 17 CH 3 | nonadecane | |
| C 20 H 42 | CH 3 (CH 2 ) 18 CH 3 | eicosane | |
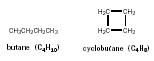
Hydrocarbons that contain one or more triple bonds are called alkynes, and is the name ending is "–yne." A 2-carbon alkyne is therefore named ethyne. (However, the compound is often referred to by its common name, which is acetylene. )
Compounds that contain double or triple bonds are said to be "unsaturated"—because they are not "saturated" with hydrogen atoms. Unsaturated compounds are reactive materials that readily add hydrogen when heated over a catalyst such as nickel. The reverse reaction also occurs. Heating ethane with steam is an important commercial process for making ethene (or ethylene). This is an important commercial process called "steam cracking."
When a 6-carbon ring contains 2 double bonds, it is called cyclohexadiene, but when it has 3 double bonds, it is not called cyclohexatriene; this is because a 6-carbon ring with three double bonds takes on a special kind of stability. The double bonds become completely conjugated and no longer behave as double bonds. The ring, known as a "benzene ring," is said to be aromatic.
The removal of a hydrogen atom from a hydrocarbon molecule leaves an alkyl group that readily attaches to a functional group, or forms a branch on a hydrocarbon chain. The groups are named after the corresponding hydrocarbons. For example, CH 3 – is named methyl; CH 3 CH 2 –, ethyl; CH 2 = CH–, ethenyl; CH 3 CH 2 CH 2 –, propyl; and so on. A benzene ring from which a hydrogen atom has been removed is often referred to as a phenyl. The branched molecules shown here would be given names as follows

Hydrocarbons are obtained primarily from fossil fuels—especially petroleum and natural gas. Natural gas is a mixture that is largely methane mixed with varying amounts of ethane and other light hydrocarbons, while petroleum is a complex mixture of many different hydrocarbons. Coal, the other fossil fuel, is a much more complicated material from which many kinds of organic compounds, some of them hydrocarbons, can be obtained.
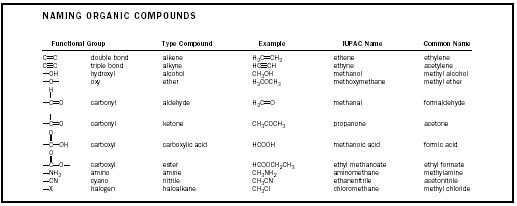
Functional Groups
Alkane molecules are rather unreactive (except for being very flammable), but alkenes react with many other substances. When a drop of bromine is added to an alkene, for example, the deep orange color of the bromine immediately disappears as the bromine adds across the double bond to form a dibromo derivative. The double bond is called a "functional group" because its presence in a molecule causes reactivity at that particular site. There are a dozen or so functional groups that appear frequently in organic compounds. Some of the most common ones are listed in Table 2. The same molecule may contain several functional groups. Aspirin, for example, is both a carboxylic acid and an ester , and cholesterol is an alkene as well as an alcohol.Isomerism
Isomers are molecules with the same molecular formula but different structures. There is only one structure for methane, ethane, or propane; but butane, C 4 H 10 , can have either of two different structure:
In a conjugated system, there are alternating double and single bonds,
allowing electrons to flow back and forth. Molecules that contain such
conjugated systems are said to be stabilized by
"resonance." In the benzene ring every other bond is a
double bond, all the way around the ring. This results in a special kind
of stabilization called "aromaticity," in which the
electrons are delocalized and free to travel all around the ring.
Certain ring compounds, like benzene, that contain such a conjugated
system of double and single bonds are described as
"aromatic."
Pentane has 3 isomers: pentane (or
n
-pentane), methylbutane (or
iso-
pentane), and dimethyl propane (or
neo
pentane). Hexane has 5 isomers:
hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and
2,3-dimethylbutane. Heptane has 9 different isomers, octane has 18, nonane
has 35, and decane has 75. An increase in the number of carbon atoms
greatly increases the possibilities for isomerism. There are more than
4,000 isomers of C
15
H
32
and more than 366,000 isomers of C
20
H
42
. The formula C
30
H
62
has more than 4 billion. Of course, most of them have never been isolated
as pure compounds (but could be, if there were any point in doing it).
For molecules other than hydrocarbons, still other kinds of isomers are possible. The simple formula C 2 H 6 O can represent ethyl alcohol or dimethyl ether; and C 3 H 6 O could stand for an alcohol, an ether, an aldehyde , or a ketone (among other things). The larger a molecule is, and the greater the variety of atoms and functional groups it contains, the more numerous its isomers.
There is still another kind of isomerism that stems from the existence of "right-" and "left-handed" molecules. It is sometimes referred to as optical isomerism because the molecules that make up a pair of these isomers usually differ only in the way they rotate plane polarized light.
Nomenclature
There are so many millions of organic compounds that simply finding names for them all is a major challenge. It was not until the late nineteenth century that chemists developed a logical system for naming organic compounds. Compounds had often been named according to their sources. The 1-carbon carboxylic acid, for example, was first obtained from ants, and so it was called formic acid, from the Latin word for ants ( formicae ). The 2-carbon acid was obtained from vinegar ( acetum in Latin), and was called acetic acid.To bring some order to the naming process an international meeting was held in 1892 at Geneva, Switzerland. The group later became known as the International Union of Pure and Applied Chemistry (IUPAC). Its objective was to establish a naming process that would provide each compound with a unique and systematic name. An initial set of rules was adopted at that first meeting in Geneva, and IUPAC has continued that work. Its systematic naming rules are used by organic chemists all over the world. The names of the alkanes form the basis for the system, with functional groups usually being indicated with appropriate suffixes. Some examples are given in Table 2.
Organic Reactions
Organic chemistry is concerned with the many compounds of carbon, their names, their isomers, and their properties, but it is mostly concerned with their reactions. Organic chemists have developed a huge array of chemical reactions that can convert one organic compound to another. Some reactions involve addition of one molecule to another; some involve decomposition of molecules; some involve substitution of one atom or group by another; and some even involve the rearrangement of molecules, with some atoms moving into new positions. Some reactions require energy in the form of heat or radiation; and some require a special kind of catalyst or some sort
Table 2.
| NAMING ORGANIC COMPOUNDS | |||||
| Functional Group | Type Compound | Example | IUPAC Name | Common Name | |
| C=C | double bond | alkene | H 2 C=CH 2 | ethene | ethylene |
| C≡C | triple bond | alkyne | HC≡CH | ethyne | acetylene |
| –OH | hydroxyl | alcohol | CH 3 OH | methanol | methyl alcohol |
| –O– | oxy | ether | H 3 COCH 3 | methoxymethane | methyl ether |
| carbonyl | aldehyde | H 2 C=0 | methanal | formaldehyde | |
| carbonyl | ketone | CH 3 COCH 3 | propanone | acetone | |
| carboxyl | carboxylic acid | HCOOH | methanoic acid | formic acid | |
| carboxyl | ester | HCOOCH 2 CH 3 | ethyl methanoate | ethyl formate | |
| –NH 2 | amino | amine | CH 3 NH 2 | aminomethane | methylamine |
| –CN | cyano | nitrile | CH 3 CN | ethanenitrile | acetonitrile |
| –X | halogen | haloalkane | CH 3 Cl | chloromethane | methyl chloride |
Organic reactions can often give remarkable control as to what products should be formed. Adding water to propene for example, produces 2-propanol in the presence of acid, but it yields 1-propanol if treated first with B 2 H 6 and then H 2 O 2 in the presence of base.
Future Sources of Organic Chemicals
Fossil fuels have been our primary natural source for many organic chemicals for more than a century, but our fossil fuel resources are finite, and they are being rapidly depleted (especially oil and gas). What will be our sources of organic materials in the future? Since fossil fuels are nonrenewable resources, it is believed that the twenty-first century will see a shift toward greater dependence on renewable raw materials. The largest U.S. chemical company has a goal of becoming 25 percent based on renewable resources by 2010. It is already producing 1,3-propanediol from cornstarch using a gene-tailored E. coli bacterium. This diol is used in Du Pont's fiber Sorona, which is said to combine the best features of both polyester and nylon fibers. Succinic acid and polyhydroxybutyrate are also obtainable from renewable crops, and the list of such renewable raw materials is destined to grow. For example, ethylene (or ethene), CH 2 =CH 2 , which is a highly important commercial chemical used in making many industrial chemicals and polymers, is presently made by steam cracking of ethane obtained from oil or natural gas; but ethylene can also be made by dehydration of ethyl alcohol made by fermentation of sugar. Efforts are even being made to use biowaste materials, such as corn husks, nutshells, and wood chips as industrial raw materials.Analytical Tools
Organic chemists often need to examine products for identification, purity analysis, or structure determination. There are some marvelous tools available to help them do these things. Chromatography , spectroscopy , and crystallography are especially widely used in organic chemistry.Column chromatography, gas chromatography, and liquid chromatography are all important methods for separating mixtures of organic compounds. Spectroscopic tools include ultraviolet (UV), infrared (IR), nuclear magnetic resonance (NMR), and mass spectroscopy (MS), each capable of providing a different kind of information about an organic compound. Although it is limited to substances that can be prepared as pure crystals, x-ray crystallography is probably the ultimate tool for determining molecular structure.
No comments:
Post a Comment