Nuclear Magnetic Resonance Spectroscopy
Over the past fifty years nuclear magnetic resonance spectroscopy, commonly referred to as nmr, has become the preeminent technique for determining the structure of organic compounds. Of all the spectroscopic methods, it is the only one for which a complete analysis and interpretation of the entire spectrum is normally expected. Although larger amounts of sample are needed than for mass spectroscopy, nmr is non-destructive, and with modern instruments good data may be obtained from samples weighing less than a milligram. To be successful in using nmr as an analytical tool, it is necessary to understand the physical principles on which the methods are based.
The nuclei of many elemental isotopes have a characteristic spin (I). Some nuclei have integral spins (e.g. I = 1, 2, 3 ....), some have fractional spins (e.g. I = 1/2, 3/2, 5/2 ....), and a few have no spin, I = 0 (e.g. 12C, 16O, 32S, ....). Isotopes of particular interest and use to organic chemists are 1H, 13C, 19F and 31P, all of which have I = 1/2. Since the analysis of this spin state is fairly straightforward, our discussion of nmr will be limited to these and other I = 1/2 nuclei.
|
For a table of nuclear spin characteristics Click Here. |
|---|
|
1. A spinning charge generates a magnetic field, as shown by the animation on the right. The resulting spin-magnet has a magnetic moment (μ) proportional to the spin. |
 |
| 2. In the presence of an external magnetic field (B0), two spin states exist, +1/2 and -1/2. The magnetic moment of the lower energy +1/2 state is aligned with the external field, but that of the higher energy -1/2 spin state is opposed to the external field. Note that the arrow representing the external field points North. |
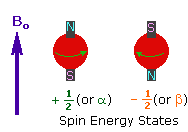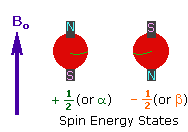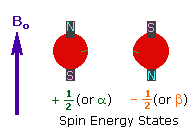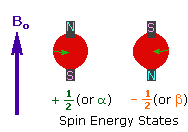 |
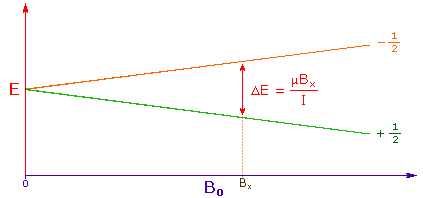
| 3. The difference in energy between the two spin states is dependent on the external magnetic field strength, and is always very small. The following diagram illustrates that the two spin states have the same energy when the external field is zero, but diverge as the field increases. At a field equal to Bx a formula for the energy difference is given (remember I = 1/2 and μ is the magnetic moment of the nucleus in the field). | |
 | |
|---|---|
| Strong magnetic fields are necessary for nmr spectroscopy. The international unit for magnetic flux is the tesla (T). The earth's magnetic field is not constant, but is approximately 10-4
T at ground level. Modern nmr spectrometers use powerful magnets having
fields of 1 to 20 T. Even with these high fields, the energy difference
between the two spin states is less than 0.1 cal/mole. To put this in
perspective, recall that infrared transitions involve 1 to 10 kcal/mole
and electronic transitions are nearly 100 time greater. For nmr purposes, this small energy difference (ΔE) is usually given as a frequency in units of MHz (106 Hz), ranging from 20 to 900 Mz, depending on the magnetic field strength and the specific nucleus being studied. Irradiation of a sample with radio frequency (rf) energy corresponding exactly to the spin state separation of a specific set of nuclei will cause excitation of those nuclei in the +1/2 state to the higher -1/2 spin state. Note that this electromagnetic radiation falls in the radio and television broadcast spectrum. Nmr spectroscopy is therefore the energetically mildest probe used to examine the structure of molecules. The nucleus of a hydrogen atom (the proton) has a magnetic moment μ = 2.7927, and has been studied more than any other nucleus. The previous diagram may be changed to display energy differences for the proton spin states (as frequencies) by mouse clicking anywhere within it. | |
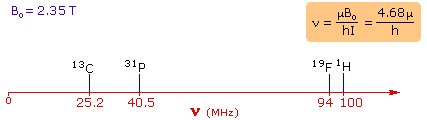
| 4. For spin 1/2 nuclei the energy difference between the two spin states at a given magnetic field strength will be proportional to their magnetic moments. For the four common nuclei noted above, the magnetic moments are: 1H μ = 2.7927, 19F μ = 2.6273, 31P μ = 1.1305 & 13C μ = 0.7022. These moments are in nuclear magnetons, which are 5.05078•10-27 JT-1. The following diagram gives the approximate frequencies that correspond to the spin state energy separations for each of these nuclei in an external magnetic field of 2.35 T. The formula in the colored box shows the direct correlation of frequency (energy difference) with magnetic moment (h = Planck's constant = 6.626069•10-34 Js). | |
 | |
2. Proton NMR Spectroscopy
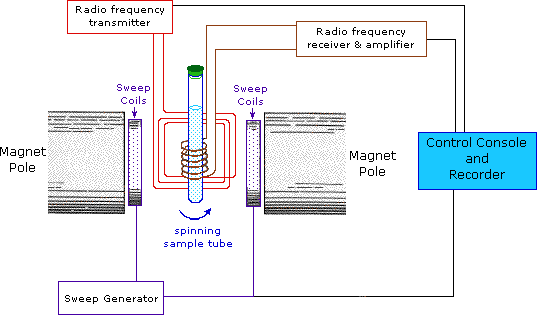
This important and well-established application of nuclear magnetic resonance will serve to illustrate some of the novel aspects of this method. To begin with, the nmr spectrometer must be tuned to a specific nucleus, in this case the proton. The actual procedure for obtaining the spectrum varies, but the simplest is referred to as the continuous wave (CW) method. A typical CW-spectrometer is shown in the following diagram. A solution of the sample in a uniform 5 mm glass tube is oriented between the poles of a powerful magnet, and is spun to average any magnetic field variations, as well as tube imperfections. Radio frequency radiation of appropriate energy is broadcast into the sample from an antenna coil (colored red). A receiver coil surrounds the sample tube, and emission of absorbed rf energy is monitored by dedicated electronic devices and a computer. An nmr spectrum is acquired by varying or sweeping the magnetic field over a small range while observing the rf signal from the sample. An equally effective technique is to vary the frequency of the rf radiation while holding the external field constant.
|
For a description of the pulse Fourier transform technique, preferred by most spectroscopists over the older CW method, Click Here. |
|---|
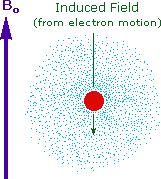
Why should the proton nuclei in different compounds behave differently in the nmr experiment ?
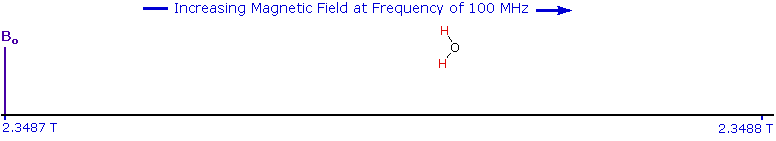
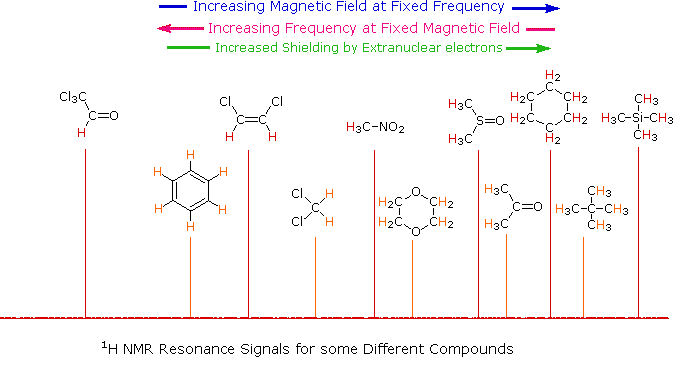
The answer to this question lies with the electron(s) surrounding the proton in covalent compounds and ions. Since electrons are charged particles, they move in response to the external magnetic field (Bo) so as to generate a secondary field that opposes the much stronger applied field. This secondary field shields the nucleus from the applied field, so Bo must be increased in order to achieve resonance (absorption of rf energy). As illustrated in the drawing on the right, Bo must be increased to compensate for the induced shielding field. In the upper diagram, those compounds that give resonance signals at the higher field side of the diagram (CH4, HCl, HBr and HI) have proton nuclei that are more shielded than those on the lower field (left) side of the diagram.
The magnetic field range displayed in the above diagram is very small compared with the actual field strength (only about 0.0042%). It is customary to refer to small increments such as this in units of parts per million (ppm). The difference between 2.3487 T and 2.3488 T is therefore about 42 ppm. Instead of designating a range of nmr signals in terms of magnetic field differences (as above), it is more common to use a frequency scale, even though the spectrometer may operate by sweeping the magnetic field. Using this terminology, we would find that at 2.34 T the proton signals shown above extend over a 4,200 Hz range (for a 100 MHz rf frequency, 42 ppm is 4,200 Hz). Most organic compounds exhibit proton resonances that fall within a 12 ppm range (the shaded area), and it is therefore necessary to use very sensitive and precise spectrometers to resolve structurally distinct sets of hydrogen atoms within this narrow range. In this respect it might be noted that the detection of a part-per-million difference is equivalent to detecting a 1 millimeter difference in distances of 1 kilometer.
Chemical Shift
One method of solving this problem is to report the location of an nmr signal in a spectrum relative to a reference signal from a standard compound added to the sample. Such a reference standard should be chemically unreactive, and easily removed from the sample after the measurement. Also, it should give a single sharp nmr signal that does not interfere with the resonances normally observed for organic compounds. Tetramethylsilane, (CH3)4Si, usually referred to as TMS, meets all these characteristics, and has become the reference compound of choice for proton and carbon nmr.
Since the separation (or dispersion) of nmr signals is magnetic field dependent, one additional step must be taken in order to provide an unambiguous location unit. This is illustrated for the acetone, methylene chloride and benzene signals by clicking on the previous diagram. To correct these frequency differences for their field dependence, we divide them by the spectrometer frequency (100 or 500 MHz in the example), as shown in a new display by again clicking on the diagram. The resulting number would be very small, since we are dividing Hz by MHz, so it is multiplied by a million, as shown by the formula in the blue shaded box. Note that νref is the resonant frequency of the reference signal and νsamp is the frequency of the sample signal. This operation gives a locator number called the Chemical Shift, having units of parts-per-million (ppm), and designated by the symbol δ Chemical shifts for all the compounds in the original display will be presented by a third click on the diagram.
The compounds referred to above share two common characteristics:
• The compounds are all liquids, save for neopentane which boils at 9 °C and is a liquid in an ice bath.
|
For the properties of some common nmr solvents Click Here. |
|---|
From the previous discussion and examples we may deduce that one factor contributing to chemical shift differences in proton resonance is the inductive effect. If the electron density about a proton nucleus is relatively high, the induced field due to electron motions will be stronger than if the electron density is relatively low. The shielding effect in such high electron density cases will therefore be larger, and a higher external field (Bo) will be needed for the rf energy to excite the nuclear spin. Since silicon is less electronegative than carbon, the electron density about the methyl hydrogens in tetramethylsilane is expected to be greater than the electron density about the methyl hydrogens in neopentane (2,2-dimethylpropane), and the characteristic resonance signal from the silane derivative does indeed lie at a higher magnetic field. Such nuclei are said to be shielded. Elements that are more electronegative than carbon should exert an opposite effect (reduce the electron density); and, as the data in the following tables show, methyl groups bonded to such elements display lower field signals (they are deshielded). The deshielding effect of electron withdrawing groups is roughly proportional to their electronegativity, as shown by the left table. Furthermore, if more than one such group is present, the deshielding is additive (table on the right), and proton resonance is shifted even further downfield.
Proton Chemical Shifts of Methyl Derivatives
|
Proton Chemical Shifts (ppm)
|
|---|
Proton Chemical Shift Ranges* | ||
|---|---|---|
| Low Field Region | 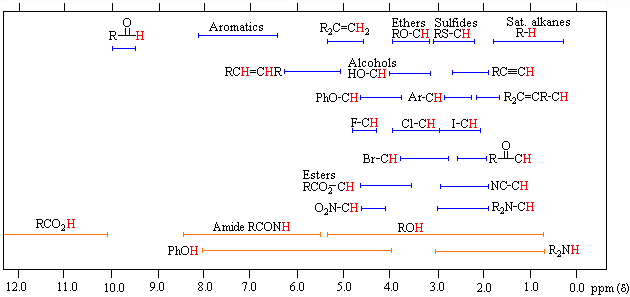 |
High Field Region |
| * For samples in CDCl3 solution. The δ scale is relative to TMS at δ = 0. | ||
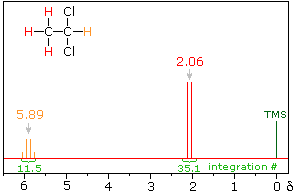
Signal Strength
Hint: When evaluating relative signal strengths, it is useful to set the smallest integration to unity and convert the other values proportionally.
Hydroxyl Proton Exchange and the Influence of Hydrogen Bonding
R-O-H + D2O  R-O-D + D-O-H R-O-D + D-O-H |
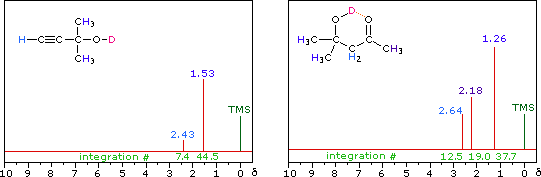 |
| i) The chemical shift of the hydroxyl hydrogen of an alcohol varies with concentration. Very dilute solutions of 2-methyl-2-propanol, (CH3)3COH, in carbon tetrachloride solution display a hydroxyl resonance signal having a relatively high-field chemical shift (< 1.0 δ ). In concentrated solution this signal shifts to a lower field, usually near 2.5 δ. | ||
| ii) The more acidic hydroxyl group of phenol
generates a lower-field resonance signal, which shows a similar
concentration dependence to that of alcohols. OH resonance signals for
different percent concentrations of phenol in chloroform-d are shown in
the following diagram (C-H signals are not shown). | ||
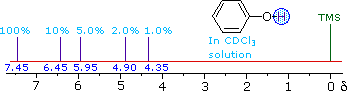 | ||
|---|---|---|
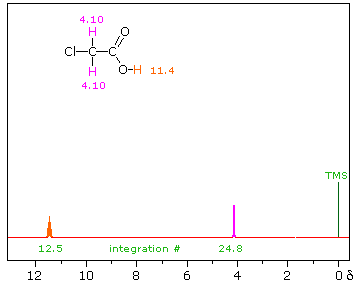
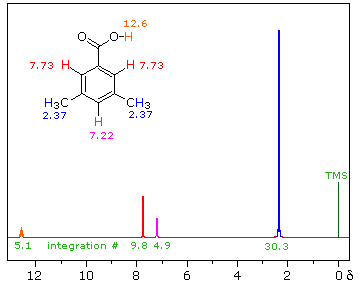
| iii) Because of their favored hydrogen-bonded dimeric association, the hydroxyl proton of carboxylic acids displays a resonance signal significantly down-field of other functions. For a typical acid it appears from 10.0 to 13.0 δ and is often broader than other signals. The spectra shown below for chloroacetic acid (left) and 3,5-dimethylbenzoic acid (right) are examples. | ||
| ||
| iv) Intramolecular hydrogen bonds, especially those defining a six-membered ring, generally display a very low-field proton resonance. The case of 4-hydroxypent-3-ene-2-one (the enol tautomer of 2,4-pentanedione) not only illustrates this characteristic, but also provides an instructive example of the sensitivity of the nmr experiment to dynamic change. In the nmr spectrum of the pure liquid, sharp signals from both the keto and enol tautomers are seen, their mole ratio being 4 : 21 (keto tautomer signals are colored purple). Chemical shift assignments for these signals are shown in the shaded box above the spectrum. The chemical shift of the hydrogen-bonded hydroxyl proton is δ 14.5, exceptionally downfield. We conclude, therefore, that the rate at which these tautomers interconvert is slow compared with the inherent time scale of nmr spectroscopy. | ||
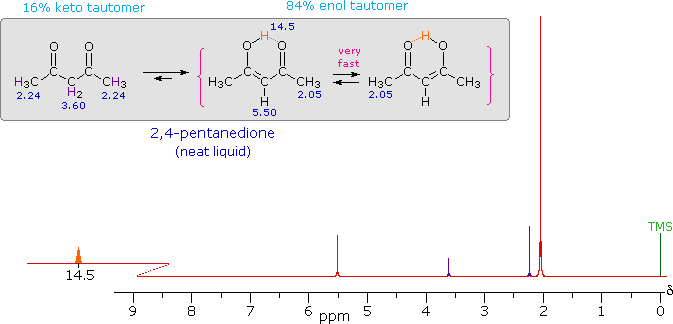 | ||
| Two structurally equivalent structures may be drawn for the
enol tautomer (in magenta brackets). If these enols were slow to
interconvert, we would expect to see two methyl resonance signals
associated with each, one from the allylic methyl and one from the
methyl ketone. Since only one strong methyl signal is observed, we must
conclude that the interconversion of the enols is very fast-so fast that
the nmr experiment detects only a single time-averaged methyl group
(50% α-keto and 50% allyl). |
Although hydroxyl protons have been the focus of this discussion, it should be noted that corresponding N-H groups in amines and amides also exhibit hydrogen bonding nmr shifts, although to a lesser degree. Furthermore, OH and NH groups can undergo rapid proton exchange with each other; so if two or more such groups are present in a molecule, the nmr spectrum will show a single signal at an average chemical shift. For example, 2-hydroxy-2-methylpropanoic acid, (CH3)2C(OH)CO2H, displays a strong methyl signal at δ 1.5 and a 1/3 weaker and broader OH signal at δ 7.3 ppm. Note that the average of the expected carboxylic acid signal (ca. 12 ) and the alcohol signal (ca. 2 ) is 7. Rapid exchange of these hydrogens with heavy water, as noted above, would cause the low field signal to disappear.
|
For additional information about the influence of hydrogen bonding Click Here. |
|---|
π-Electron Functions
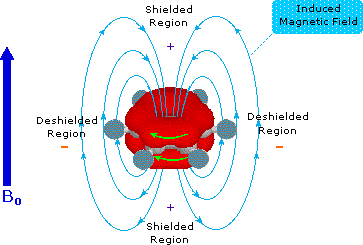
Pi-electrons are more polarizable than are sigma-bond electrons, as addition reactions of electrophilic reagents to alkenes testify. Therefore, we should not be surprised to find that field induced pi-electron movement produces strong secondary fields that perturb nearby nuclei. The pi-electrons associated with a benzene ring provide a striking example of this phenomenon, as shown below. The electron cloud above and below the plane of the ring circulates in reaction to the external field so as to generate an opposing field at the center of the ring and a supporting field at the edge of the ring. This kind of spatial variation is called anisotropy, and it is common to nonspherical distributions of electrons, as are found in all the functions mentioned above. Regions in which the induced field supports or adds to the external field are said to be deshielded, because a slightly weaker external field will bring about resonance for nuclei in such areas. However, regions in which the induced field opposes the external field are termed shielded because an increase in the applied field is needed for resonance. Shielded regions are designated by a plus sign, and deshielded regions by a negative sign.
The anisotropy of some important unsaturated functions will be displayed by clicking on the benzene diagram below. Note that the anisotropy about the triple bond nicely accounts for the relatively high field chemical shift of ethynyl hydrogens. The shielding & deshielding regions about the carbonyl group have been described in two ways, which alternate in the display.
|
For additional examples of chemical shift variation near strongly anisotropic groups Click Here. |
|---|
Solvent Effects
|
CDCl3 | C6D6 | CD3COCD3 | CD3SOCD3 | CD3C≡N | D2O | ||
|---|---|---|---|---|---|---|---|---|
| (CH3)3C–O–CH3 C–CH3 O–CH3 |
1.19 3.22 | 1.07 3.04 | 1.13 3.13 | 1.11 3.03 | 1.14 3.13 | 1.21 3.22 | ||
| (CH3)3C–O–H C–CH3 O–H |
1.26 1.65 | 1.05 1.55 | 1.18 3.10 | 1.11 4.19 | 1.16 2.18 | --- --- | ||
| C6H5CH3 CH3 C6H5 |
2.36 7.15-7.20 | 2.11 7.00-7.10 | 2.32 7.10-7.20 | 2.30 7.10-7.15 | 2.33 7.15-7.30 | --- --- | ||
| (CH3)2C=O | 2.17 | 1.55 | 2.09 | 2.09 | 2.08 | 2.22 |
The second noteworthy change is seen in the spectrum of tert-butanol in DMSO, where the hydroxyl proton is shifted 2.5 ppm down-field from where it is found in dilute chloroform solution. This is due to strong hydrogen bonding of the alcohol O–H to the sulfoxide oxygen, which not only de-shields the hydroxyl proton, but secures it from very rapid exchange reactions that prevent the display of spin-spin splitting. Similar but weaker hydrogen bonds are formed to the carbonyl oxygen of acetone and the nitrogen of acetonitrile. A useful application of this phenomenon is described elsewhere in this text.
Spin-Spin Interactions
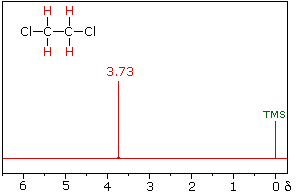 |  | |
| 1,2-dichloroethane | 1,1-dichloroethane |
|---|
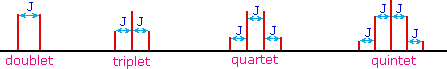
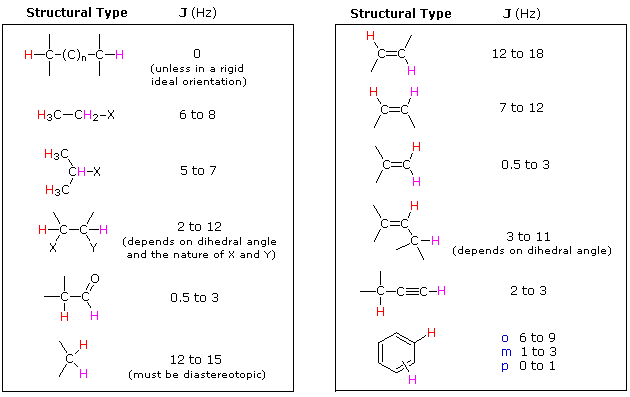
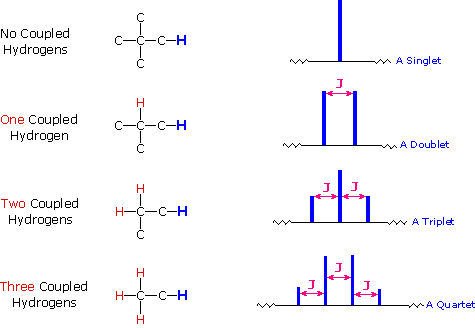
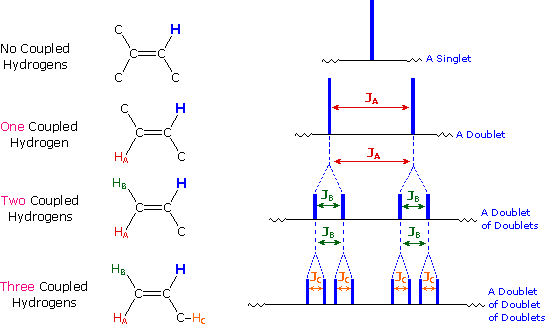
The splitting patterns found in various spectra are easily recognized, provided the chemical shifts of the different sets of hydrogen that generate the signals differ by two or more ppm. The patterns are symmetrically distributed on both sides of the proton chemical shift, and the central lines are always stronger than the outer lines. The most commonly observed patterns have been given descriptive names, such as doublet (two equal intensity signals), triplet (three signals with an intensity ratio of 1:2:1) and quartet (a set of four signals with intensities of 1:3:3:1). Four such patterns are displayed in the following illustration. The line separation is always constant within a given multiplet, and is called the coupling constant (J). The magnitude of J, usually given in units of Hz, is magnetic field independent.
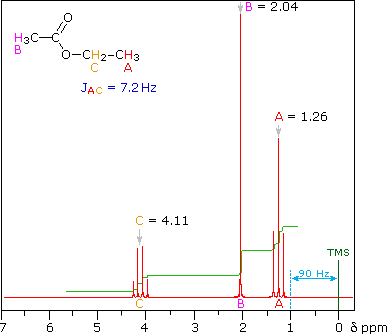
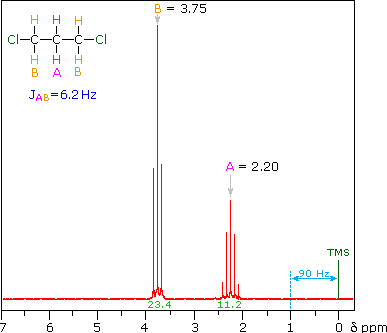
The splitting patterns shown above display the ideal or "First-Order" arrangement of lines. This is usually observed if the spin-coupled nuclei have very different chemical shifts (i.e. Δν is large compared to J). If the coupled nuclei have similar chemical shifts, the splitting patterns are distorted (second order behavior). In fact, signal splitting disappears if the chemical shifts are the same. Two examples that exhibit minor 2nd order distortion are shown below (both are taken at a frequency of 90 MHz). The ethyl acetate spectrum on the left displays the typical quartet and triplet of a substituted ethyl group. The spectrum of 1,3-dichloropropane on the right demonstrates that equivalent sets of hydrogens may combine their influence on a second, symmetrically located set.
Even though the chemical shift difference between the A and B protons in the 1,3-dichloroethane spectrum is fairly large (140 Hz) compared with the coupling constant (6.2 Hz), some distortion of the splitting patterns is evident. The line intensities closest to the chemical shift of the coupled partner are enhanced. Thus the B set triplet lines closest to A are increased, and the A quintet lines nearest B are likewise stronger. A smaller distortion of this kind is visible for the A and C couplings in the ethyl acetate spectrum.
 |  |
|
For additional examples of Second Order splitting patterns Click Here. |
|---|
What causes this signal splitting, and what useful information can be obtained from it ?
If an atom under examination is perturbed or influenced by a nearby nuclear spin (or set of spins), the observed nucleus responds to such influences, and its response is manifested in its resonance signal. This spin-coupling is transmitted through the connecting bonds, and it functions in both directions. Thus, when the perturbing nucleus becomes the observed nucleus, it also exhibits signal splitting with the same J. For spin-coupling to be observed, the sets of interacting nuclei must be bonded in relatively close proximity (e.g. vicinal and geminal locations), or be oriented in certain optimal and rigid configurations. Some spectroscopists place a number before the symbol J to designate the number of bonds linking the coupled nuclei (colored orange below). Using this terminology, a vicinal coupling constant is 3J and a geminal constant is 2J.
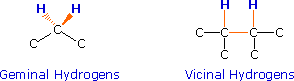
The following general rules summarize important requirements and characteristics for spin 1/2 nuclei :
2) Nuclei separated by three or fewer bonds (e.g. vicinal and geminal nuclei ) will usually be spin-coupled and will show mutual spin-splitting of the resonance signals (same J's), provided they have different chemical shifts. Longer-range coupling may be observed in molecules having rigid configurations of atoms.
3) The magnitude of the observed spin-splitting depends on many factors and is given by the coupling constant J (units of Hz). J is the same for both partners in a spin-splitting interaction and is independent of the external magnetic field strength.
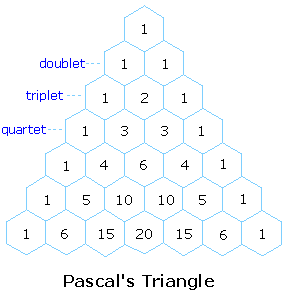
4) The splitting pattern of a given nucleus (or set of equivalent nuclei) can be predicted by the n+1 rule, where n is the number of neighboring spin-coupled nuclei with the same (or very similar) Js. If there are 2 neighboring, spin-coupled, nuclei the observed signal is a triplet ( 2+1=3 ); if there are three spin-coupled neighbors the signal is a quartet ( 3+1=4 ). In all cases the central line(s) of the splitting pattern are stronger than those on the periphery. The intensity ratio of these lines is given by the numbers in Pascal's triangle. Thus a doublet has 1:1 or equal intensities, a triplet has an intensity ratio of 1:2:1, a quartet 1:3:3:1 etc. To see how the numbers in Pascal's triangle are related to the Fibonacci series click on the diagram.
 < < |
|---|
To make use of a calculator that predicts first order splitting patterns Click Here. This application was developed at Colby College.
|
For additional information about spin-spin coupling Click Here. |
|---|
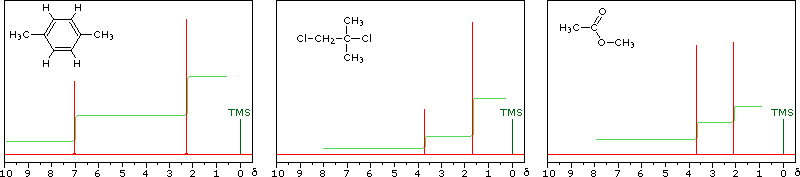
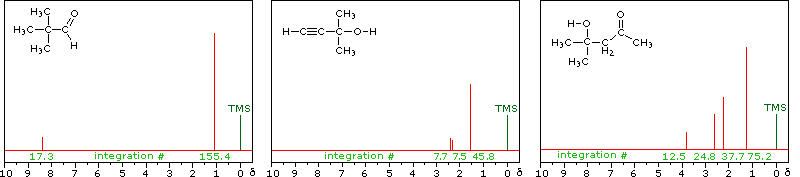
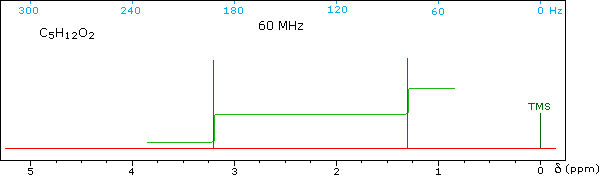
Some Examples
Although the first four cases are relatively simple, keep in mind that the integration values provide ratios, not absolute numbers. In two cases additional information from infrared spectroscopy is provided. When you have made an assignment you may check your answer by clicking on the spectrum itself. In the sixth example, a similar constitutional isomer cannot be ruled out by the data given.
|
For a challenging problem having many spin couplings Click Here. |
|---|
3. Carbon NMR Spectroscopy
The power and usefulness of 1H nmr spectroscopy as a tool for structural analysis should be evident from the past discussion. Unfortunately, when significant portions of a molecule lack C-H bonds, no information is forthcoming. Examples include polychlorinated compounds such as chlordane, polycarbonyl compounds such as croconic acid, and compounds incorporating triple bonds (structures below, orange colored carbons).
| Many obstacles needed to be overcome before carbon nmr emerged as a routine tool : i) As noted, the abundance of 13C in a sample is very low (1.1%), so higher sample concentrations are needed. ii) The 13C nucleus is over fifty times less sensitive than a proton in the nmr experiment, adding to the previous difficulty. iii) Hydrogen atoms bonded to a 13C atom split its nmr signal by 130 to 270 Hz, further complicating the nmr spectrum. |
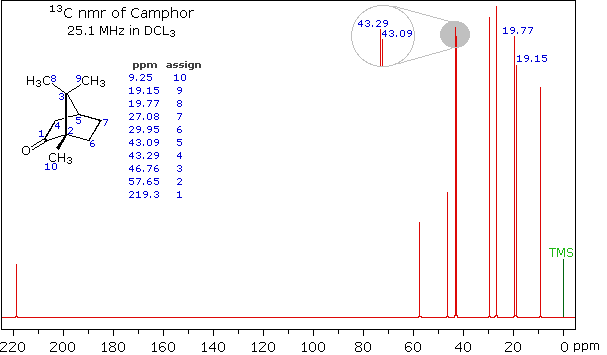
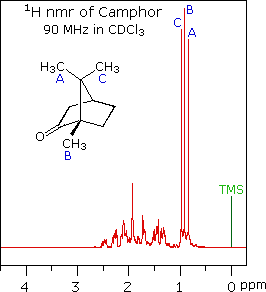
When acquired in this manner, the carbon nmr spectrum of a compound displays a single sharp signal for each structurally distinct carbon atom in a molecule (remember, the proton couplings have been removed). The spectrum of camphor, shown on the left below, is typical. Furthermore, a comparison with the 1H nmr spectrum on the right illustrates some of the advantageous characteristics of carbon nmr. The dispersion of 13C chemical shifts is nearly twenty times greater than that for protons, and this together with the lack of signal splitting makes it more likely that every structurally distinct carbon atom will produce a separate signal. The only clearly identifiable signals in the proton spectrum are those from the methyl groups. The remaining protons have resonance signals between 1.0 and 2.8 ppm from TMS, and they overlap badly thanks to spin-spin splitting.
 |  |
|---|
13C Chemical Shift Ranges* | ||
|---|---|---|
| Low Field Region | 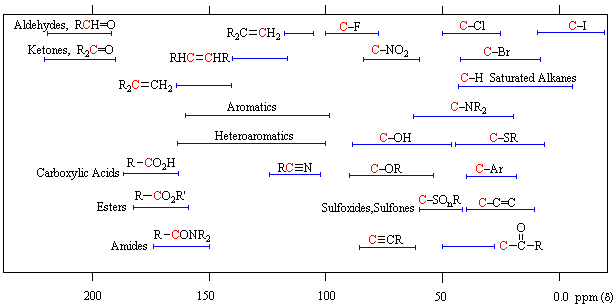 |
High Field Region |
| * For samples in CDCl3 solution. The δ scale is relative to TMS at δ=0. | ||
No comments:
Post a Comment