|
ALKENES and POTASSIUM MANGANATE(VII)
This page looks at the reaction of the carbon-carbon double bond in
alkenes such as ethene with potassium manganate(VII) solution (potassium
permanganate solution). Oxidation of alkenes with cold dilute potassium manganate(VII) solution Experimental details Alkenes react with potassium manganate(VII) solution in the cold. The colour change depends on whether the potassium manganate(VII) is used under acidic or alkaline conditions. If the potassium manganate(VII) solution is acidified with dilute sulphuric acid, the purple solution becomes colourless. If the potassium manganate(VII) solution is made slightly alkaline (often by adding sodium carbonate solution), the purple solution first becomes dark green and then produces a dark brown precipitate. Chemistry of the reaction We'll look at the reaction with ethene. Other alkenes react in just the same way. Manganate(VII) ions are a strong oxidising agent, and in the first instance oxidise ethene to ethane-1,2-diol (old name: ethylene glycol). Looking at the equation purely from the point of view of the organic reaction: 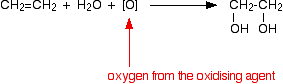 | |
|
Note: This type of equation is quite commonly used in organic chemistry. Oxygen written in square brackets is taken to mean "oxygen from an oxidising agent". The reason for this is that a more normal equation tends to obscure the organic change in a mass of other detail - as you will find below! The full equations are given below, although you probably won't need them. | |
|
The full equation depends on the conditions. Under acidic conditions, the manganate(VII) ions are reduced to manganese(II) ions. | |
|
Note: If you want to know how to write equations for redox reactions like this you could follow this link, and explore in the redox section of this site. Use the BACK button (or HISTORY file or GO menu) on your browser to return to this page later. | |
Under alkaline conditions, the manganate(VII) ions are first reduced to green manganate(VI) ions . . .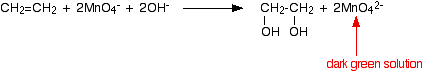 . . . and then further to dark brown solid manganese(IV) oxide (manganese dioxide). 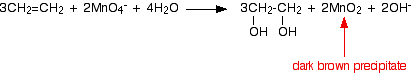 This last reaction is also the one you would get if the reaction was done under neutral conditions. You will notice that there are neither hydrogen ions nor hydroxide ions on the left-hand side of the equation. | |
|
Note: You might possibly remember that further up the page it says that potassium manganate(VII) is often made slightly alkaline by adding sodium carbonate solution. Where are the hydroxide ions in this? Carbonate ions react with water to some extent to produce hydrogencarbonate ions and hydroxide ions. It is the presence of these hydroxide ions that gives sodium carbonate solution its pH in the 10 - 11 region. | |
|
Using the reaction to test for carbon-carbon double bonds If an organic compound reacts with dilute alkaline potassium manganate(VII) solution in the cold to give a green solution followed by a dark brown precipitate, then it may contain a carbon-carbon double bond. But equally it could be any one of a large number of other compounds all of which can be oxidised by manganate(VII) ions under alkaline conditions. The situation with acidified potassium manganate(VII) solution is even worse because it has a tendency to break carbon-carbon bonds. It reacts destructively with a large number of organic compounds and is rarely used in organic chemistry. You could use alkaline potassium manganate(VII) solution if, for example, all you had to do was to find out whether a hydrocarbon was an alkane or an alkene - in other words, if there was nothing else present which could be oxidised. It isn't a useful test. Bromine water is far more clear cut. | |
|
Note: You will find details of the use of bromine water in testing for carbon-carbon double bonds in the page about the reactions of alkenes with halogens. | |
|
Oxidation of alkenes with hot concentrated acidified potassium manganate(VII) solution
This is where it gets complicated! Check with your syllabus to see
whether you need to know about it before you go any further. This
section was written to cover a statement in the Cambridge International
(CIE) A level syllabus. The problem The diols, such as ethane-1,2-diol, which are the products of the reaction with cold dilute potassium manganate(VII), are themselves quite easily oxidised by manganate(VII) ions. That means that the reaction won't stop at this point unless the potassium manganate(VII) solution is very dilute, very cold, and preferably not under acidic conditions. If you are using hot concentrated acidified potassium manganate(VII) solution, what you finally end up with depends on the arrangement of groups around the carbon-carbon double bond. Writing a structural formula to represent any alkene The formula below represents a general alkene. In organic chemistry, the symbol R is used to represent hydrocarbon groups or hydrogen in a formula when you don't want to talk about specific compounds. If you use the symbol more than once in a formula (as here), the various groups are written as R1, R2, etc. In this particular case, the double bond is surrounded by four such groups, and these can be any combination of same or different - so they could be 2 hydrogens, a methyl and an ethyl, or 1 hydrogen and 3 methyls, or 1 hydrogen and 1 methyl and 1 ethyl and 1 propyl, or any other combination you can think of. In other words, this formula represents every possible simple alkene: 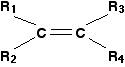 The acidified potassium manganate(VII) solution oxidises the alkene by breaking the carbon-carbon double bond and replacing it with two carbon-oxygen double bonds. 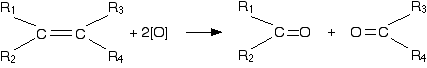 | |
|
Warning: The rest of this page is going to look quite difficult, because it talks in some detail about compounds you probably won't have studied yet. It may be best just to go through this quickly for now, and then come back to it later on after you have studied aldehydes and ketones. | |
|
What happens next? If both attached R groups in the products are alkyl groups Carbonyl compounds which have two hydrocarbon groups attached to the carbonyl group are called ketones. Ketones aren't that easy to oxidise, and so there is no further action. (But see note in red below.) If the groups attached either side of the original carbon-carbon double bond were the same, then you would end up with a single ketone. If they were different, then you would end up with a mixture of two. For example: 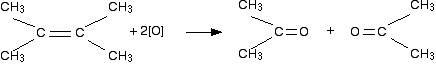 What would you get if there was a methyl and an ethyl group on both sides of the original carbon-carbon double bond? Again, you would get a single ketone formed - in this case, butanone. If you aren't sure about this, draw the structures and see. | |
|
Important: This last section is a gross over-simplification for the purposes of the CIE A level syllabus. In practice, ketones are oxidised by potassium manganate(VII) solution under these conditions. The reaction is untidy and results in breaking carbon-carbon bonds either side of the carbonyl group. If you are doing CIE, then you will have to learn this as stated above. If you are doing anything else, you probably shouldn't be wasting your time reading this anyway. Potassium manganate(VII) is such a devastating oxidising agent that it is rarely used in organic chemistry. Check your syllabus! | |
|
If a product has one hydrocarbon group and one hydrogen For example, suppose the first stage of the reaction was: 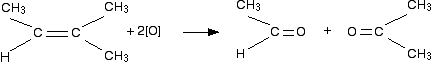 Aldehydes are readily oxidised to give carboxylic acids, containing the -COOH group. So this time, the reaction will go on a further step to give ethanoic acid, CH3COOH. 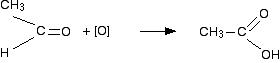 The overall effect of the potassium manganate(VII) on this kind of alkene is therefore: 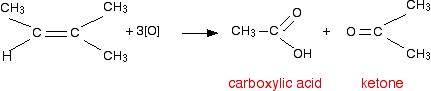 Play around with this until you are happy about it. Draw a number of alkenes, all of which have a hydrogen attached at both ends of the carbon-carbon double bond. Vary the alkyl groups - sometimes the same on each end of the double bond, sometimes different. Oxidise them to form the acids, and see what you get. If a product has two hydrogens but no hydrocarbon group You might have expected that this would produce methanoic acid, as in the equation: 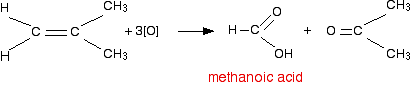 So the equation in a case like this might be, for example: 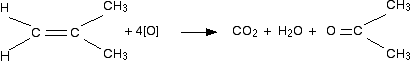 If there were two hydrogens at both ends of the double bond (in other words, if you had ethene), then all you would get would be carbon dioxide and water. Summary Think about both ends of the carbon-carbon double bond separately, and then combine the results afterwards.
Working back from the results helps you to work out the structure of the alkene. For example, the alkene C4H8 has three structural isomers: 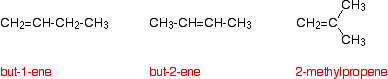
| |
Monday, 24 June 2013
ALKENES and POTASSIUM MANGANATE(VII)
Subscribe to:
Post Comments (Atom)
Our trained chemists work on custom projects designed specifically for the needs of each client. 1-dectyl-2,3-methylimidazolium perfluorobutanesulfonate
ReplyDelete