Physical Properties of Organic Compounds
Boiling Point and Vapor Pressure
The transition between liquid and gas is very important for organic liquids. It is useful for purification through distillation, and is responsible for evaporation. Finally, without some vapor pressure, no compound could reach your nose to produce those wonderful or terrible smells we associate with organic chemistry.The vapor pressure is the actual pressure of vapor over a liquid. Measurable at all temperatures, it is smaller at lower temperatures, and increases with higher temperatures. Eventually, the vapor pressure will meet or exceed atmospheric pressure, and bubbles will form in the liquid—a process we call “boiling.” The temperature at which the vapor pressure equals atmospheric pressure is the official boiling point.
Let’s think about the physical process involved in boiling. The figure below shows molecules (represented by “X”) in the liquid on the left, and in the gas on the right:
Physical process:
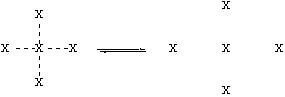
X--X is composite of polar, H-bonding, and Van der Waals forces.
The big change on boiling is that the intermolecular forces (X---X) are broken, and not replaced by anything. [Note, however, that the molecule X stays intact, and the covalent bonds are not broken.] This means that the stronger the intermolecular forces (X—X) are, the harder it will be to disrupt them. This will yield a lower vapor pressure at a given temperature, and higher boiling point. These intermolecular forces will be a composite of polar, H-bonding, and Van der Waals forces.
Predicting Boiling Points
Even for professional chemists, it is very difficult to predict the boiling point of a single compound. However, it is not too difficult in simple cases to predict the relative boiling points of two compounds (that is, which compound boils at the higher temperature).| Boiling points of homologous series of compounds with 8 functional groups. Note the trend towards higher boiling points as compounds get longer within a series, and the four classes of functional groups by strength of intermolecular interaction. | 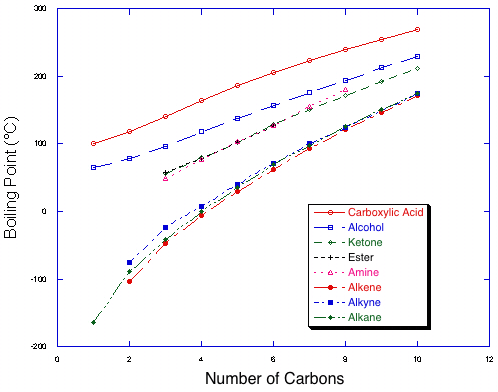 |
Within a series, there is a steady increase in boiling points as compounds get larger. This is due to an increase in surface area, and therefore in van der Waals interactions. The addition of one or two carbons will not cause much of a rise in boiling point, but it will be noticeable and can add up with large changes in size. Note also that the lines get somewhat closer as the molecules get larger, because as one adds more alkane content to the molecule, the molecules increasingly resemble alkanes.
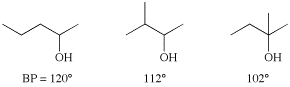
Another important factor in boiling point prediction relates to the difference between branched and linear carbon skeletons. Since branching allows the compound to be more compact, it reduces surface area, reducing the van der Waals interactions, and therefore reducing the boiling point. Note the effects of branching on the following three alcohols:
No comments:
Post a Comment