|
SOME BERYLLIUM CHEMISTRY UNTYPICAL OF GROUP 2
This page describes and explains three examples from beryllium
chemistry where it behaves differently from the rest of Group 2. In
fact, there are several similarities between beryllium and aluminium in
Group 3. This is known as a diagonal relationship and is discussed on the page about electronegativity. Beryllium chloride is covalent The facts Physical properties Beryllium chloride, BeCl2, melts at 405°C and boils at 520°C. That compares with 714°C and 1412°C for magnesium chloride. Notice how much dramatically lower the boiling point of beryllium chloride is compared with magnesium chloride. The much higher boiling point of magnesium chloride is what you might expect from the strong forces between the positive and negative ions present. Because its boiling point is much lower, it follows that beryllium chloride can't contain ions - it must be covalent. On the other hand, the melting point is quite high for such a small covalent molecule. There must be something more complicated going on! Reaction with water Beryllium chloride reacts vigorously and exothermically with water with the evolution of acidic, steamy hydrogen chloride gas. This is typical of covalent chlorides. In the first instance, it reacts to give hydrated beryllium ions, [Be(H2O)4]2+, and chloride ions. But the hydrated beryllium ions (called tetraaquaberyllium ions) are quite strongly acidic. The small beryllium ion at the centre attracts the electrons in the bonds towards itself, and that makes the hydrogen atoms in the water even more positive than they usually are. If the solution is hot and concentrated (as it is likely to be if you add water to solid beryllium chloride - a very exothermic reaction), chloride ions can remove one or more of these hydrogen ions to produce hydrogen chloride gas. All the other ionic chlorides in Group 2 dissolve in water without any obvious reaction. | |
|
Note: There is actually a very small amount of reaction between anhydrous magnesium chloride and water, although you wouldn't notice it. Magnesium chloride isn't quite as purely ionic as we sometimes pretend! Follow this link if you are interested in exploring the naming of complex ions. . . . or this one for detailed explanations of why complex ions similar to the beryllium one are acidic. | |
|
The structure of beryllium chloride As a gas . . . Beryllium chloride, BeCl2, is a linear molecule with all three atoms in a straight line. Showing only the outer electrons: 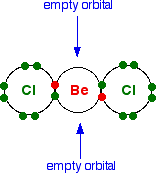 As a solid . . . If it had this same simple structure as a solid, you would expect the melting point to be much lower than it actually is. It is a very small molecule, and so the intermolecular attractions would be expected to be fairly weak. | |
|
Note: If you aren't sure about intermolecular forces (Van der Waals forces) you could follow this link to find out more about them. Use the BACK button on your browser to return to this page. | |
In the solid, the BeCl2 molecules polymerise to make long
chains. They do this by forming coordinate bonds (dative covalent
bonds) between lone pairs on chlorine atoms and adjacent beryllium
atoms. The diagram shows a simple dimer - the start of the
polymerisation process. | |
|
Note: This is exactly the same as the coordinate bonding in aluminium chloride. If you aren't happy about coordinate (dative covalent) bonding, it would pay you to look at this page before you go on. Use the BACK button on your browser to return to this page. | |
You can see from the last diagram that the beryllium atoms are still
electron deficient. The process continues. The next diagram shows the
coordinate bonds in the conventional way using arrows. The arrow goes
from the atom which is supplying the pair of electrons to the atom with
the empty orbital.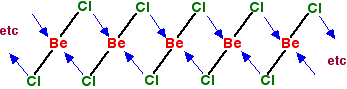 Why isn't beryllium chloride ionic? Beryllium has quite a high electronegativity compared with the rest of the Group. That means that it attracts a bonding pair of electrons towards itself more strongly than magnesium and the rest do. In order for an ionic bond to form, the beryllium has to let go of its electrons. It is too electronegative to do that. | |
|
Note: The trends in electronegativity in Group 2 are discussed on another page. That page looks at the way the electons are arranged in the beryllium-chlorine bond compared with the magnesium-chlorine bond. Use the BACK button on your browser to return to this page - or come back via the Group 2 menu. | |
|
Beryllium forms 4-coordinated complex ions
Some simple background Although beryllium doesn't normally form simple ions, Be2+, it does form ions in solution. In these, the beryllium ion becomes attached to four water molecules to give a complex ion with the formula [Be(H2O)4]2+. The ion is said to be 4-coordinated, or to have a coordination number of 4, because there are four water molecules arranged around the central beryllium. Many hydrated metal ions are 6-coordinated. For example, magnesium ions in solution exist as [Mg(H2O)6]2+. The water molecules in these ions are attached to the central metal ion via coordinate bonds (dative covalent bonds). One of the lone pairs on each water molecule is used to form a bond with an empty orbital in the metal ion. Each time one of these bonds is formed, energy is released, and the ion becomes more stable. It would seem logical for the metal ion to form as many bonds like this as it possibly can. | |
|
Note: If you aren't happy about coordinate bonding you must follow this link before you go on. You will find the bonding in hydrated metal ions discussed in some detail on that page. Use the BACK button on your browser to return to this page. | |
|
Why does beryllium only attach four water molecules? The hydration of beryllium The problem is that there has to be somewhere that the lone pairs on the water molecules can attach to. Beryllium has the electronic structure 1s22s2. It is helpful to draw this as an "electrons-in-boxes" diagram: 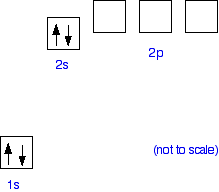 | |
|
Note: If you aren't happy about orbitals you really ought to follow this link before you go on. You may want to explore further in that part of the site as well. Unless you understand exactly what this electrons-in-boxes diagram is about, you won't be able to make sense of what is coming up next. | |
|
When beryllium forms a 2+ ion it loses the 2 electrons in the 2s orbital. That leaves the 2-level completely empty. The 2-level orbitals reorganise themselves (hybridise) to make four equal orbitals, each of which can accept a lone pair of electrons from a water molecule. In the next diagram the 1s electrons have been left out. They aren't relevant to the bonding. 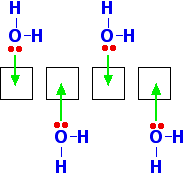 Notice that once four water molecules have bonded in this way, there isn't any more space available at the bonding level. All the empty orbitals from the original beryllium ion are being used. The water molecules arrange themselves to get as far apart as possible - which is pointing towards the corners of a tetrahedron. The ion therefore has a tetrahedral shape. The hydration of magnesium You might think that magnesium would behave just the same, but at the 3-level there are 3d orbitals available as well as 3s and 3p. When the magnesium ion is formed, it leaves empty 3s, 3p and 3d orbitals. When that ion is hydrated, it uses the 3s orbital, all three of the 3p orbitals and two of the 3d orbitals. These are reorganised to leave a total of six empty orbitals which are then used for bonding. 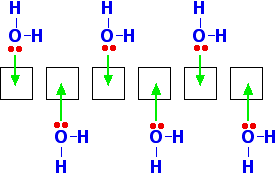 What about the other ions in Group 2? As the ions get bigger, there is less tendency for them to form proper coordinate bonds with water molecules. The ions become so big that they aren't sufficiently attractive to the lone pairs on the water molecules to form formal bonds - instead the water molecules tend to cluster more loosely around the positive ions. Where they do form coordinate bonds with the water, however, they will be 6-coordinated just like the magnesium. Beryllium hydroxide is amphoteric Amphoteric means that it can react with both acids and bases to form salts. The other Group 2 hydroxides The other hydroxides of the Group 2 metals are all basic. They react with acids to form salts. For example: Calcium hydroxide reacts with dilute hydrochloric acid to give calcium chloride and water. Beryllium hydroxide Beryllium hydroxide reacts with acids, forming solutions of beryllium salts. For example: But it also reacts with bases such as sodium hydroxide solution. Beryllium hydroxide reacts with the sodium hydroxide to give a colourless solution of sodium tetrahydroxoberyllate. This contains the complex ion, [Be(OH)4]2-. The name describes this ion. Tetra means four; hydroxo refers to the OH groups; beryllate shows that the beryllium is present in a negative ion. The "ate" ending always shows that the ion is negative. A simple explanation of what is happening You need to think about where the beryllium hydroxide came from in the first place. It would probably have been made by adding sodium hydroxide solution to a solution of a beryllium salt like beryllium sulphate. Remember that beryllium ions in solution exist as the hydrated ion, [Be(H2O)4]2+. The beryllium has such a strongly polarising effect on the water molecules that hydrogen ions are very easily removed from them. The sodium hydroxide solution contains hydroxide ions which are powerful bases. If you add just the right amount of sodium hydroxide solution, you get a precipitate of what is normally called "beryllium hydroxide" - but which is a shade more complicated than that! The product (other than water) is a neutral complex, and it is covalently bonded. All that has happened to the original complex ion is that two hydrogen ions have been removed from the water molecules. You get a precipitate of the neutral complex because of the lack of charge on it. There isn't enough attraction between this neutral complex and water molecules to bring it into solution. What happens if you add an acid to this? The hydrogen ions that were originally removed are simply replaced. The precipitate dissolves as the original hydrated beryllium ion is re-formed. What happens if you add a base? Adding more hydroxide ions to the neutral complex pulls more hydrogen ions off the water molecules to give the tetrahydroxoberyllate ion: The beryllium hydroxide dissolves because the neutral complex is converted into an ion which will be sufficiently attracted to water molecules. Why doesn't this happen with, for example, calcium hydroxide? Calcium hydroxide is truly ionic - and contains simple hydroxide ions, OH-. These react with hydrogen ions from an acid to form water - and so the hydroxide reacts with acids. However, there isn't any equivalent to the neutral complex. Adding more hydroxide ions from a base has no effect because they haven't got anything to react with. | |
Saturday, 29 June 2013
SOME BERYLLIUM CHEMISTRY UNTYPICAL OF GROUP 2
Labels:
Inorganic Chemistry
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment