|
ALKENES and SULPHURIC ACID
This page looks at the reaction of the carbon-carbon double bond in
alkenes such as ethene with concentrated sulphuric acid. It includes
the conversion of the product into an alcohol. The addition of sulphuric acid to alkenes The reaction with ethene Alkenes react with concentrated sulphuric acid in the cold to produce alkyl hydrogensulphates. Ethene reacts to give ethyl hydrogensulphate. The structure of the product molecule is sometimes written as CH3CH2HSO4, but the version in the equation is better because it shows how all the atoms are linked up. You may also find it written as CH3CH2OSO3H. 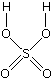 Confused by all this? Don't be!
Confused by all this? Don't be!All you need to do is to learn the structure of sulphuric acid. A hydrogen from the sulphuric acid joins on to one of the carbon atoms, and the rest joins on to the other one. Make sure that you can see how the structure of the sulphuric acid relates to the various ways of writing the formula for the product. | |
|
Important! Learn this structure for sulphuric acid. Sketch it over and over again until you can't possibly get it wrong. Follow this link if you want the mechanism for this reaction. Use the BACK button on your browser to return to this page. | |
|
The reaction with propene This is typical of the reaction with unsymmetrical alkenes. An unsymmetrical alkene has different groups at either end of the carbon-carbon double bond. If sulphuric acid adds to an unsymmetrical alkene like propene, there are two possible ways it could add. You could end up with one of two products depending on which carbon atom the hydrogen attaches itself to. However, in practice, there is only one major product. 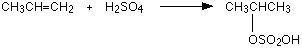 This is in line with Markovnikov's Rule which says:
Notice that only the hydrogens directly attached to the carbon atoms at either end of the double bond count. The ones in the CH3 group are totally irrelevant. | |
|
Warning! Markovnikov's Rule is a useful guide for you to work out which way round to add something across a double bond, but it isn't the reason why things add that way. As a general principle, don't quote Markovnikov's Rule in an exam unless you are specifically asked for it. You can find more about this in the mechanism section of this site. You will find the mechanism for this reaction discussed in detail if you follow this link. Use the BACK button (or HISTORY file or GO menu, if you have to explore several pages) on your browser if you want to return to this page. | |
|
Using these reactions to make alcohols
Making ethanol Ethene is passed into concentrated sulphuric acid to make ethyl hydrogensulphate (as above). The product is diluted with water and then distilled. The water reacts with the ethyl hydrogensulphate to produce ethanol which distils off. Making propan-2-ol More complicated alkyl hydrogensulphates react with water in exactly the same way. For example: Notice that the position of the -OH group is determined by where the HSO4 group was attached. You get propan-2-ol rather than propan-1-ol because of the way the sulphuric acid originally added across the double bond in propene. Using these reactions These reactions were originally used as a way of manufacturing alcohols from alkenes in the petrochemical industry. These days, alcohols like ethanol or propan-2-ol tend to be manufactured by direct hydration of the alkene because it is cheaper and easier | |
Monday, 24 June 2013
ALKENES and SULPHURIC ACID
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment