REACTIONS OF THE GROUP 1 ELEMENTS WITH OXYGEN AND CHLORINE
This page mainly looks at the reactions of the Group 1 elements
(lithium, sodium, potassium, rubidium and caesium) with oxygen -
including the simple reactions of the various kinds of oxides formed.
It also deals very briefly with the reactions of the elements with
chlorine.
The Reactions with Air or Oxygen
General
These are all very reactive metals and have to be stored out of
contact with air to prevent their oxidation. Reactivity increases as
you go down the Group.
Lithium, sodium and potassium are stored in oil. (Lithium in fact
floats on the oil, but there will be enough oil coating it to give it
some protection. It is, anyway, less reactive than the rest of the
Group.)
Rubidium and caesium are normally stored in sealed glass tubes to
prevent air getting at them. They are stored either in a vacuum or in
an inert atmosphere of, say, argon. The tubes are broken open when the
metal is used.
Depending on how far down the Group you are, different kinds of oxide
are formed when the metals burn (details below). Reaction with oxygen
is just a more dramatic version of the reaction with air.
Lithium is unique in the Group because it also reacts with the nitrogen in the air to form lithium nitride (again, see below).
Details for the individual metals
Lithium
Lithium burns with a strongly red-tinged flame if heated in air. It
reacts with oxygen in the air to give white lithium oxide. With pure
oxygen, the flame would simply be more intense.
 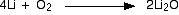
For the record, it also reacts with the nitrogen in the air to give
lithium nitride. Lithium is the only element in this Group to form a
nitride in this way.
 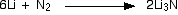
|
|
Note: You will find the reason why lithium forms a nitride on the page about reactions of Group 2 elements with air or oxygen. You will find what you want about 3/4 of the way down that page.
Lithium's reactions are often rather like those of the Group 2 metals. There is a diagonal relationship between lithium and magnesium. You will find this discussed on the page about electronegativity.
Use the BACK button on your browser to return to this page from either of these links.
|
Sodium
Small pieces of sodium burn in air with often little more than an
orange glow. Using larger amounts of sodium or burning it in oxygen
gives a strong orange flame. You get a white solid mixture of sodium
oxide and sodium peroxide.
The equation for the formation of the simple oxide is just like the lithium one.
 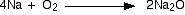
The peroxide equation is:
 
Potassium
Small pieces of potassium heated in air tend to just melt and turn
instantly into a mixture of potassium peroxide and potassium superoxide
without any flame being seen. Larger pieces of potassium burn with a
lilac flame.
The equation for the formation of the peroxide is just like the sodium one above:
 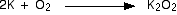
. . . and for the superoxide:
 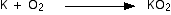
|
|
Note: Potassium peroxide and superoxide are described
as being somewhere between yellow and orange depending on what source
you look at. I have a bit of a problem with this, because over my
teaching career I have heated potassium in air many times and, if memory
serves correctly, it always leaves a greyish white film on the bit of
porcelain you are heating it on. I don't recall ever seeing it yellow
or orange!
The
formula for a peroxide doesn't look too stange, because most people are
familiar with the similar formula for hydrogen peroxide. The formula
for a superoxide always looks wrong! There is more about these oxides
later on.
|
Rubidium and caesium
Both metals catch fire in air and produce superoxides, RbO2 and CsO2. The equations are the same as the equivalent potassium one.
|
|
Note: In a lifetime in teaching chemistry, I have
never actually handled (or even seen in real life!) either of these
metals. I haven't even seen video or film clips of them being burnt.
That means that I don't have much confidence in this next bit.
|
Both superoxides are described in most sources as being either orange
or yellow. One major web source describes rubidium superoxide as being
dark brown on one page and orange on another!
I don't know what the flames look like either. You can't necessarily
be sure that the flame that a metal burns with will be the same as the
flame colour of its compounds.
Why are different oxides formed as you go down the Group?
- Lithium (and to some extent sodium) form simple oxides, X2O, which contain the common O2- ion.
- Sodium (and to some extent potassium) form peroxides, X2O2, containing the more complicated O22- ion (discussed below).
- Potassium, rubidium and caesium form superoxides, XO2. The structure of the superoxide ion, O2-, is too difficult to discuss at this level, needing a good knowledge of molecular orbital theory to make sense of it.
The more complicated ions aren't stable in the presence of a small positive ion. Consider the peroxide ion, for example.
The peroxide ion, O22- looks like this:
The covalent bond between the two oxygen atoms is relatively weak. |
Reaction with dilute acids
These simple oxides all react with an acid to give a salt and water.
For example, sodium oxide will react with dilute hydrochloric acid to
give colourless sodium chloride solution and water.
 
The peroxides, X2O2
Reaction with water
If the reaction is done ice cold (and the temperature controlled so
that it doesn't rise even though these reactions are strongly
exothermic), a solution of the metal hydroxide and hydrogen peroxide is
formed.
 
If the temperature increases (as it inevitably will unless the
peroxide is added to water very, very, very slowly!), the hydrogen
peroxide produced decomposes into water and oxygen. The reaction can be
very violent overall.
Reaction with dilute acids
These reactions are even more exothermic than the ones with water. A
solution containing a salt and hydrogen peroxide is formed. The
hydrogen peroxide will decompose to give water and oxygen if the
temperature rises - again, it is almost impossible to avoid this.
Another potentially violent reaction!
 
The superoxides, XO2
Reaction with water
This time, a solution of the metal hydroxide and hydrogen peroxide is
formed, but oxygen gas is given off as well. Once again, these are
strongly exothermic reactions and the heat produced will inevitably
decompose the hydrogen peroxide to water and more oxygen. Again
violent!
 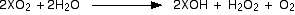
Reaction with dilute acids
Again, these reactions are even more exothermic than the ones with
water. A solution containing a salt and hydrogen peroxide is formed
together with oxygen gas. The hydrogen peroxide will again decompose to
give water and oxygen as the temperature rises. Violent!
 
The Reactions of the elements with Chlorine
This is included on this page because of the similarity in appearance
between the reactions of the Group 1 metals with chlorine and with
oxygen.
Sodium, for example, burns with an intense orange flame in chlorine
in exactly the same way that it does in pure oxygen. The rest also
behave the same in both gases.
In each case, there is a white solid residue which is the simple
chloride, XCl. There is nothing in any way complicated about these
reactions!
 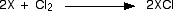 |

No comments:
Post a Comment