|
ESSENTIAL BONDING THEORY FOR UV-VISIBLE ABSORPTION SPECTROMETRY
This page takes an introductory look at two areas of bonding theory
needed for a proper understanding of how organic compounds absorb some
of the UV or visible light that passes through them. It looks simply at anti-bonding orbitals, and what is meant by conjugation in compounds and how it contributes to the delocalisation of electrons. It may well be that this page goes in to more detail than your syllabus expects, but it isn't all that difficult and you can't really understand what is going on without it. It would, however, be worth checking your syllabus to find out exactly how much you need to remember. | |
|
Note: If you are studying for a UK-based exam for 16 - 18 year olds, and haven't got a copy of your syllabus, you can find out how to get one by following this link. | |
|
Anti-bonding orbitals
Bonding and anti-bonding orbitals in a simple hydrogen molecule I am assuming that you know how a simple covalent bond between two atoms forms. Half-filled atomic orbitals on each atom overlap in space to form a new orbital (a molecular orbital) containing both electrons. | |
|
Note: If this is new to you, then you are starting in the wrong place! The simplest place to start on this site is probably to explore a few pages of the organic bonding menu. You need to read at least the first two pages from that menu. Use the BACK button (or HISTORY file or GO menu) on your browser to return here later. | |
In the case of two hydrogen atoms, each has one electron in the 1s
orbital. These come together to make a new orbital surrounding both of
the hydrogen nuclei. The molecule holds together because both nuclei are strongly attracted to this same pair of electrons. This most simple of bonds is called a sigma bond - a sigma bond is one where the electron pair is most likely to be found on the line between the two nuclei. However . . . This is all a bit of a simplification! Molecular orbital theory demands that if you start with two atomic orbitals, you must end up with two molecular orbitals - and we seem to be only producing one. A second molecular orbital is formed, but in most cases (including the hydrogen molecule) it is left empty of electrons. It is described as an anti-bonding orbital. The anti-bonding orbital has a quite different shape and energy from the bonding orbital. The next diagram shows the relative shapes and energies of the various atomic and molecular orbitals when two hydrogen atoms combine. 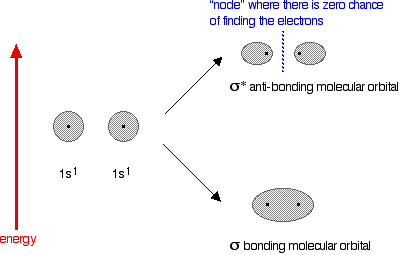 Notice that when a bonding orbital forms, it is at a lower energy than the original atoms. Energy is released when the bonding orbital is formed, and the hydrogen molecule is more energetically stable than the original atoms. However, an anti-bonding orbital is less energetically stable than the original atoms. A bonding orbital is stable because of the attractions between the nuclei and the electrons. In an anti-bonding orbital there are no equivalent attractions - instead you get repulsions. There is very little chance of finding the electrons between the two nuclei - and in fact half-way between the nuclei there is zero chance of finding them. There is nothing to stop the two nuclei from repelling each other apart. So in the hydrogen case, both of the electrons go into the bonding orbital, because that produces the greatest stability - more stable than having separate atoms, and a lot more stable than having the electrons in the anti-bonding orbital. Why doesn't helium form an He2 molecule? This is just to reinforce the ideas above. You might reasonably say that helium can't form an He2 molecule because it doesn't have any unpaired electrons to share. Fine! But let's also look at it from the point of molecular orbital theory. The diagram for helium is just a small modification of the last one. 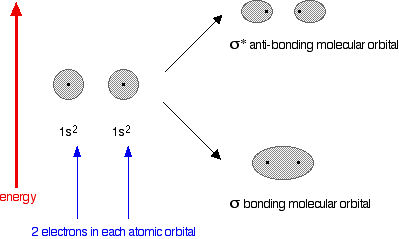 But any gain in energetic stability due to the formation of the bonding orbitals would be countered by the loss of energetic stability because of the anti-bonding ones. There is no energetic advantage in He2 forming - and so it doesn't. Anti-bonding orbitals in double bonds You are probably familiar with a picture of the double bond in ethene shown as: 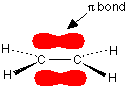 | |
|
Note: Bonds are drawn in various ways to suggest how the atoms are arranged in 3 dimensions. A bond shown with a normal unbroken line is in the plane of the screen or paper. A dotted line shows a bond going away from you. A wedge shows one coming out towards you. | |
|
The pi bond shown in red is, of course, a normal bonding orbital. It
was formed by sideways overlap between a half-filled p-orbital on each
of the two carbon atoms. Remember that the two red shapes shown in the
diagram are part of the same pi bonding orbital. But if you overlap two atomic orbitals, you must get two molecular orbitals according to molecular orbital theory. The second one is an anti-bonding pi orbital - and we never draw it under normal circumstances. The anti-bonding pi orbital is (just like the anti-bonding sigma one) at a higher energy than the bonding orbital - and so isn't used to hold electrons. Both of the electrons in the pi bond are found in the pi bonding orbital. | |
|
Note: If you aren't happy about the diagram of an ethene molecule, read the page about bonding in ethene in the organic bonding section of the site. Use the BACK button on your browser to return here later. | |
|
Summarising the relative energies of various kinds of orbitals The next diagram gives a general impression of how the energies of various types of orbital relate to each other in the sort of compounds we will be looking at when we try to explain the absorption of light. It is not to scale. You will find that a new sort of orbital has crept into the diagram - labelled "n" (for non-bonding). The sort of non-bonding orbitals that we will be interested in contain lone pairs of electrons on, for example, oxygen, nitrogen and halogen atoms. So . . . think of non-bonding orbitals as those containing lone pairs of electrons at the bonding level. | |
|
Note: Be careful not to confuse non-bonding with anti-bonding - they mean totally different things. The non-bonding orbitals we are interested in contain a lone pair of electrons - a perfectly stable, filled orbital. Anti-bonding orbitals are normally empty and lower the stability of a compound if they contain electrons. If in doubt, go back and re-read the stuff about anti-bonding orbitals, and be sure that you can see that they have nothing to do with lone pairs. | |
 The energy gaps between these levels determine the frequency (or wavelength) of the light absorbed, and those gaps will be different in different compounds. This is covered in detail on another page. Conjugation We are going to leave explaining what conjugation is for a while - it is necessary to look at some more bonding first. The simple ethene double bond To understand about conjugated double bonds, you first need to be sure that you understand simple double bonds. Ethene contains a simple double bond between two carbon atoms, but the two parts of this bond are different. Part of it is a simple sigma bond formed from end-to-end overlap between orbitals on each carbon atom, and part is caused by sideways overlap between a p-orbital on each carbon. | |
|
Note: If you aren't confident about this, read the page mentioned above about bonding in ethene in the organic bonding section of the site. It is essential that you feel happy about this - otherwise you won't make sense of what comes next. Use the BACK button on your browser to return here later. | |
The important diagram is the one leading up to the formation of the pi bond - where the two p-orbitals are overlapping sideways: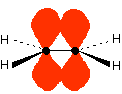  Bonding in buta-1,3-diene Buta-1,3-diene has the structure: 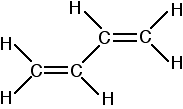 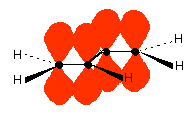 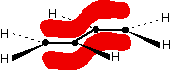 The interaction of the two double bonds with each other to produce a delocalised system of pi electrons over all four atoms is known as conjugation. Conjugation in this context literally means "joining together". In reality, if you start by overlapping four atomic orbitals, you will end up with four molecular orbitals. The four electrons will go into the two lowest energy of these - two in each. That means that you get two pi bonding orbitals. We just draw one of these for simplicity - the other one has a different shape. There are also two pi anti-bonding orbitals, but these are normally empty. For most purposes, we ignore these entirely - although not for this topic because energy from light can promote electrons from a pi bonding orbital into one of the anti-bonding orbitals (as you will see on the next page). Recognising conjugated double bonds in a molecule You can recognise the presence of conjugated double bonds in a molecule containing more than one double bond because of the presence of alternating double and single bonds. The double bonds don't have to be always between carbon atoms. All of the following molecules contain conjugated double bonds, although in the last case, the conjugation doesn't extend over the whole molecule: 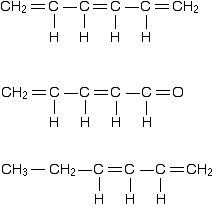 Delocalisation extending beyond conjugated double bonds Benzene rings You are almost certainly familiar with the delocalisation which occurs in a benzene ring. If you think about benzene using the Kelulé structure, you have a perfect system of alternating single and double bonds around the molecule.  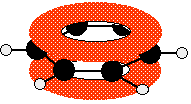 | |
|
Note: If you aren't familiar with this, you will find two pages of Chemguide dealing with the bonding in benzene. One deals with the Kelulé structure, and the other with the modern delocalised structure. You should read both of these pages starting with the one about the Kelulé structure. You will find a link to the second page at the bottom of the first one. This is likely to take you some time because you will probably have to follow up other links as well in order to fully understand the second page. Don't try to short-cut this. Use the BACK button (or more likely, the HISTORY file or GO menu) on your browser to return to this page later. | |
|
Phenylamine and phenol Delocalisation can also extend beyond pi bonds to include lone pairs on atoms like nitrogen or oxygen. Two simple examples of this are phenylamine (aniline) and phenol. Writing these using the Kekulé structure for benzene: 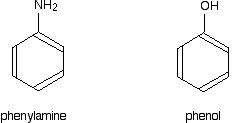  In the phenylamine case, there is a lone pair on the nitrogen atom which can overlap with the ring electrons . . . 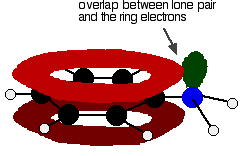 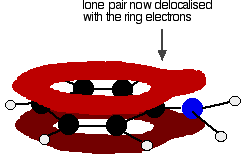 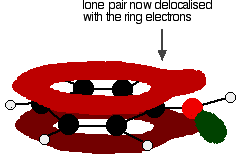 Other groups to look out for Look especially for benzene rings with groups attached which contain double bonds. Let's start with a couple of simple ones - phenylethene (styrene) and benzaldehyde. 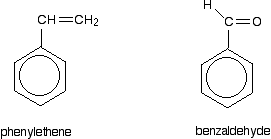 Yes, you do. You have the double bond in the side group, then a single bond, then the ring delocalisation. Looking at this in the phenylethene case, and imagining the arrangement of orbitals just before delocalisation over the side group: 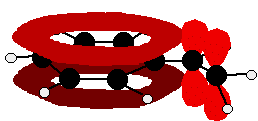 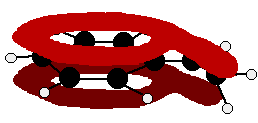 | |
|
Note: Don't panic about learning the shapes of all these delocalised structures. All that matters is that you can recognise the possibility of extensive delocalisation in them. I'm only giving you the details so that you can understand why the delocalisation arises. | |
The benzaldehyde case is very similar, except that this time instead of the CH2 group at the end there is an oxygen with two lone pairs. The delocalisation is just the same.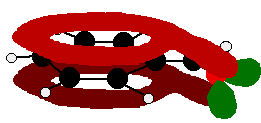 | |
|
Note: In fact because of the electronegativity of the oxygen, there is a distortion of the delocalised system towards the oxygen - but that doesn't affect the argument in any way. If you aren't happy about how I got at this structure, have a look at the page about bonding in the carbon-oxygen double bond. Use the BACK button (or the HISTORY file or GO menu) on your browser to return to this page later. | |
|
Be careful, though! Remember that to get this extended
delocalisation, any double bond in the side chain must be able to
conjugate with the ring electrons - the two bits must be close enough to
join together. Molecules like those in the next diagram don't have the delocalisation extending out into the side chain. The extra CH2 group prevents the necessary sideways overlap between the p orbitals of the double bond and the ring electrons. 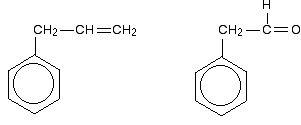 The bonding in the nitro group is surprisingly awkward to work out. It is often shown with a double bond between the nitrogen and one of the oxygens, and a co-ordinate (dative covalent) bond to the other. 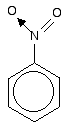 | |
|
Note: The bonding in the nitro group is like the bonding in part of a nitric acid molecule which you will find described on the page about co-ordinate (dative covalent) bonds. If you choose to follow this link, use the BACK button on your browser to return to this page later. | |
This structure is actually misleading. Both nitrogen-oxygen bonds
are identical and the group already has delocalisation. This is often
shown as: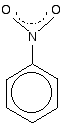 This delocalisation is just one single bond away from the ring delocalisation. You get conjugation between the two, and the delocalisation takes in the whole molecule. A case to beware of Another case we need to look at (because it occurs in a molecule we'll explore on the next page) is an SO3- group attached to a benzene ring. 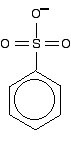 The bonding in the sulphonate group isn't at all easy to describe in orbital terms. In fact, it is most easily explained in terms of co-ordinate (dative covalent) bonds, but introducing that now is just complicating things pointlessly. I would hope that you wouldn't get asked a question about delocalisation involving this group at this level. It occurs in methyl orange (discussed on the next page), but its presence there isn't important to the main argument and it can be ignored. Summary When you are trying to work out how far delocalisation extends in a molecule, look for:
You will find lots more about this if you follow the link below to the next page. | |
Thursday, 27 June 2013
ESSENTIAL BONDING THEORY FOR UV-VISIBLE ABSORPTION SPECTROMETRY
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment