|
THE OXIDISING ABILITY OF THE GROUP 7 ELEMENTS (THE HALOGENS)
This page explores the trend in oxidising ability of the Group 7
elements (the halogens) - fluorine, chlorine, bromine and iodine. We
are going to look at the ability of one halogen to oxidise the ions of
another one, and how that changes as you go down the Group. | ||||||||||||||||||||||||||
|
Note: If you aren't comfortable with terms like oxidation and oxidising agent in terms of electron transfer, then you should explore the area of the site dealing with redox reactions before you go on. | ||||||||||||||||||||||||||
|
The facts
We are going to look at the reactions between one halogen (chlorine,
say) and the ions of another one (iodide ions, perhaps). The iodide ions
will be in a solution of a salt like sodium or potassium iodide. The
sodium or potassium ions will be spectator ions, and are completely
irrelevant to the reaction. In the chlorine and iodide ion case, the reaction would be: The iodide ions have lost electrons to form iodine molecules. They have been oxidised. The chlorine molecules have gained electrons to form chloride ions. They have been reduced. This is obviously a redox reaction in which chlorine is acting as an oxidising agent. Fluorine We'll have to exclude fluorine from this descriptive bit, because it is too strong an oxidising agent. Fluorine oxidises water to oxygen and so it is impossible to do simple solution reactions with it. Chlorine, bromine and iodine In each case, a halogen higher in the Group can oxidise the ions of one lower down. For example, chlorine can oxidise the bromide ions (in, for example, potassium bromide solution) to bromine: The bromine appears as an orange solution. As you have seen above, chlorine can also oxidise iodide ions (in, for example, potassium iodide solution) to iodine: The iodine appears either as a red solution if you are mean with the amount of chlorine you use, or as a dark grey precipitate if the chlorine is in excess. | ||||||||||||||||||||||||||
|
Note: The reason for the red solution is that iodine dissolves in potassium iodide (or other soluble iodides) by reacting to give a red ion, I3-. If the chlorine is in excess, obviously there isn't anything left for the iodine to react with, and so it remains as a dark grey precipitate. | ||||||||||||||||||||||||||
|
Bromine can only oxidise iodide ions to iodine. It isn't a strong
enough oxidising agent to convert chloride ions into chlorine. (You
have just seen exactly the reverse of that happening.) A red solution of iodine is formed (see the note above) until the bromine is in excess. Then you get a dark grey precipitate. Iodine won't oxidise any of the other halide ions (unless you happened to have some extremely radioactive and amazingly rare astatide ions - astatine is at the bottom of this Group). To summarise
Explaining the trend Whenever one of these halogens is involved in oxidising something in solution, the halogen ends up as halide ions with water molecules attached to them. Looking at all four of the common halogens: 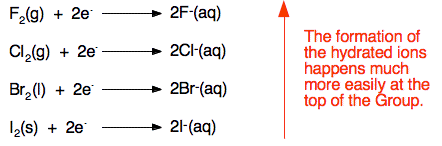 As you go down the Group, the ease with which these hydrated ions are formed falls, and so the halogens become less good as oxidising agents - less ready to take electrons from something else. The reason that the hydrated ions form less readily as you go down the Group is a fairly complicated mixture of several factors. Unfortunately, this is often over-simplified to give what is actually a faulty and misleading explanation. We'll deal with this first before giving a proper explanation. The faulty explanation This is normally given for the trend in oxidising ability of chlorine, bromine and iodine, and goes like this: How easily the element forms its ions depends on how strongly the new electrons are attracted. As the atoms get bigger, the new electrons find themselves further from the nucleus, and more and more screened from it by the inner electrons (offsetting the effect of the greater nuclear charge). The bigger atoms are therefore less good at attracting new electrons and forming ions. That sounds reasonable! What's wrong with it? What we are describing is the trend in electron affinity as you go from chlorine to bromine to iodine. Electron affinity tends to fall as you go down the Group. This is described in detail on another page. | ||||||||||||||||||||||||||
|
Note: If you haven't recently read about the electron affinities of the halogens, you ought to follow this link before you go on. Use the BACK button on your browser to return to this page. | ||||||||||||||||||||||||||
|
The snag comes if you try to expand the argument to include fluorine.
Fluorine has a much higher tendency to form its hydrated ion than
chlorine does. BUT . . . the tendency of the fluorine atom to gain an
electron is less than that of chlorine - as measured by its electron
affinity! That makes a nonsense of the whole argument. So, what is going wrong? The mistake is to look at only one part of a much more complicated process. The argument about atoms accepting electrons applies to isolated atoms in the gas state picking up electrons to make isolated ions - also in the gas state. That's not what we should be talking about. In reality:
| ||||||||||||||||||||||||||
|
Note: For the next bit, if you aren't happy about enthalpy changes, you might want to explore the energetics section of Chemguide, or my chemistry calculations book. | ||||||||||||||||||||||||||
|
The proper explanation The table below looks at how much energy is involved in each of these changes. To be sure that you understand the various terms:
You can see that the amount of heat evolved falls quite dramatically from the top to the bottom of the Group, with the biggest fall from fluorine to chlorine. Fluorine produces a lot of heat when it forms its hydrated ion, chlorine less so, and so on down the Group. | ||||||||||||||||||||||||||
|
Note: Don't forget that we are only talking about half of a redox reaction in each case. There will be other energy terms involving whatever the halogen is oxidising. Those changes will be overall endothermic. For example, if chlorine oxidises iodide ions to iodine, that half of the total reaction would need +481 kJ mol-1, giving an enthalpy change of reaction of (-592 + 481) = -111 kJ per mole of I- oxidised. | ||||||||||||||||||||||||||
|
Why is fluorine a much stronger oxidising agent than chlorine? What produces the very negative value for the enthalpy change when fluorine turns into its hydrated ions? There are two main factors. The atomisation energy of fluorine is abnormally low. This reflects the low bond enthalpy of fluorine. | ||||||||||||||||||||||||||
|
Note: The reason for fluorine's low bond enthalpy is described on another page. | ||||||||||||||||||||||||||
|
The main reason, though, is the very high hydration enthalpy of the
fluoride ion. That is because the ion is very small. There is a very
strong attraction between the fluoride ions and water molecules. The
stronger the attraction, the more heat is evolved when the hydrated ions
are formed. Why the fall in oxidising ability from chlorine to bromine to iodine? The fall in atomisation energy between these three elements is fairly slight, and would tend to make the overall change more negative as you go down the Group. The explanation doesn't lie there! It is helpful to look at the changes in electron affinity and hydration enthalpy as you go down the Group. Using the figures from the previous table:
As you go down the Group, the ions become less attractive to water molecules as they get bigger. Although the ease with which an atom attracts an electron matters, it isn't actually as important as the hydration enthalpy of the negative ion formed. The faulty explanation misses the mark even if you restrict it to chlorine, bromine and iodine | ||||||||||||||||||||||||||
Wednesday, 12 June 2013
THE OXIDISING ABILITY OF THE GROUP 7 ELEMENTS (THE HALOGENS)
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment