|
THE ACIDITY OF THE HYDROGEN HALIDES
This page looks at the acidity of the hydrogen halides - hydrogen
fluoride, hydrogen chloride, hydrogen bromide and hydrogen iodide. It
starts by describing their physical properties and how they might be
made and then explains what happens when they react with water to make
acids like hydrofluoric acid and hydrochloric acid. There is then a lengthy discussion (should you need it!) of why hydrofluoric acid is a weak acid. The hydrogen halides - background information Physical properties The hydrogen halides are colourless gases at room temperature, producing steamy fumes in moist air. Hydrogen fluoride has an abnormally high boiling point for the size of the molecule (293 K or 20°C), and could condense to a liquid on a cool day. 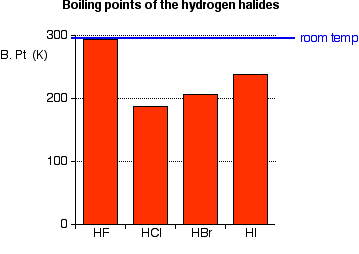 | ||||||||||||||||||||||||||
|
Note: If you aren't happy about hydrogen bonding, you ought to follow this link before you go on. Use the BACK button on your browser to return to this page. | ||||||||||||||||||||||||||
|
Fluorine is the most electronegative of all the elements and the bond
between it and hydrogen is very polar. The hydrogen atom carries quite
a lot of positive charge ( In addition, each fluorine atom has 3 very active lone pairs of electrons. Fluorine's outer electrons are at the 2-level, and the lone pairs represent small highly charged regions of space. Hydrogen bonds form between the 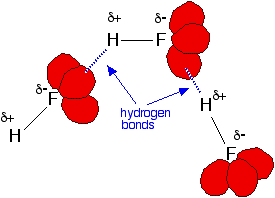 | ||||||||||||||||||||||||||
|
Note: If you aren't sure about why the electronegativity of the halogens changes as you go down the Group, you could follow this link. Use the BACK button on your browser to return to this page. | ||||||||||||||||||||||||||
|
Making the hydrogen halides There are several ways of making hydrogen halides, but the only one of interest at A' level is the reaction between an ionic halide (like sodium chloride) and an acid like concentrated phosphoric(V) acid, H3PO4, or concentrated sulphuric acid. Making hydrogen chloride You can add concentrated sulphuric acid to a solid chloride like sodium chloride in the cold. The concentrated sulphuric acid donates a hydrogen ion to a chloride ion to make hydrogen chloride. Because this is a gas, it immediately escapes from the system. The full equation for the reaction is: Sodium hydrogensulphate is also formed. Concentrated phosphoric(V) acid behaves similarly. You would again add it to solid sodium choride. Once again, as soon as any hydrogen chloride is formed, it escapes as a gas. The ionic equation is: . . . and the full one showing the formation of the salt, sodium dihydrogenphosphate(V) is: Making the other hydrogen halides All of the hydrogen halides can be made in an exactly similar way using concentrated phosphoric(V) acid. All you would need to do is swap the symbol Cl in the two equations for whichever other halogen you were interested in. The situation is more complicated with concentrated sulphuric acid. Hydrogen fluoride can be made in exactly the same way as hydrogen chloride using concentrated sulphuric acid, but hydrogen bromide and hydrogen iodide can't. The problem is that concentrated sulphuric acid is a reasonably strong oxidising agent, and as well as producing hydrogen bromide or hydrogen iodide, some of the halide ions are oxidised to bromine or iodine. This problem doesn't happen with phosphoric(V) acid because it isn't an oxidising agent. | ||||||||||||||||||||||||||
|
Note: Redox reactions involving halide ions and conc sulphuric acid are covered on a separate page. | ||||||||||||||||||||||||||
|
The acidity of the hydrogen halides
Hydrogen chloride as an acid We are going to use the Bronsted-Lowry definition of an acid as a proton donor. Hydrogen chloride is an acid because it gives protons (hydrogen ions) to other things. We are going to concentrate on its reaction with water. Hydrogen chloride gas is very soluble in water, reacting with it to produce hydrochloric acid. The familiar steamy fumes of hydrogen chloride in moist air are caused by the hydrogen chloride reacting with water vapour in the air to produce a fog of concentrated hydrochloric acid. A proton is donated from the hydrogen chloride to one of the lone pairs on a water molecule. | ||||||||||||||||||||||||||
|
Note: If you need to revise co-ordinate (dative covalent) bonding, you could follow this link. That page also describes the reaction between hydrogen chloride and ammonia - another reaction of hydrogen chloride as an acid. Use the BACK button on your browser to return to this page. | ||||||||||||||||||||||||||
|
The equation for the reaction is: The H3O+ ion is the hydroxonium ion (also known as the hydronium ion or the oxonium ion). This is the ion that we are actually talking about when we write H+(aq). When hydrogen chloride dissolves in water (to produce hydrochloric acid), almost 100% of the hydrogen chloride molecules react in this way. Hydrochloric acid is therefore a strong acid. A strong acid is one which is fully ionised in solution. Hydrobromic acid and hydriodic acid as strong acids Hydrogen bromide and hydrogen iodide dissolve in (and react with) water in exactly the same way as hydrogen chloride does. Hydrogen bromide reacts to give hydrobromic acid; hydrogen iodide gives hydriodic acid. Both of these are also strong acids. Hydrofluoric acid as an exception By contrast, although hydrogen fluoride dissolves freely in water, hydrofluoric acid is only a weak acid - similar in strength to organic acids like methanoic acid. The reason for this is quite complicated. | ||||||||||||||||||||||||||
|
Warning! Are you sure you need to know the reason for this? The explanation is far more complicated than books at this level tend to suggest. To understand it properly you need to be familiar with energy cycles, entropy, free energy and equilibrium constants. The explanation starts fairly easily, but gets harder and harder as you go on! | ||||||||||||||||||||||||||
|
Looking at the bond enthalpy of the H-F bond Because the fluorine atom is so small, the bond enthalpy (bond energy) of the hydrogen-fluorine bond is very high. Comparing all the hydrogen-halogen bond enthalpies:
It might seem reasonable, but if you dig a little deeper, that explanation falls apart! | ||||||||||||||||||||||||||
|
Note: If you aren't happy about enthalpy changes, you might want to explore the energetics section of Chemguide, or my chemistry calculations book. | ||||||||||||||||||||||||||
|
Looking at the energetics of the process from HX(g) to X-(aq) We need to consider the energetics of this sequence: 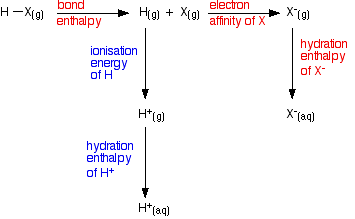 However, the terms involving the hydrogen will be the same for every hydrogen halide. So if we are just trying to draw comparisons, we only need to look at the terms shown in red in the diagram.
| ||||||||||||||||||||||||||
|
Note: There is so much discrepancy between the numbers we are using if you obtain them from different sources that you couldn't count a 10 kJ difference in the totals as being significant. These particular figures come from a variety of sources of varying reliability! | ||||||||||||||||||||||||||
|
The large bond enthalpy of the H-F bond is compensated for by the
large hydration enthalpy of the fluoride ion. There is a very strong
attraction between the very small fluoride ion and the water molecules.
This releases a lot of heat (the hydration enthalpy) when the fluoride
ion becomes wrapped in water molecules. The fact that there isn't much difference between the overall values means that we still aren't looking in the right place for the explanation of why hydrofluoric acid is weak! Looking at other attractions in the system The mistake we are making so far is in starting from the wrong place! The energy terms we have been looking at start from HX as a gas. In fact, we should be thinking of it starting in solution - but not yet reacted with the water. The proper equation we should be working from is: 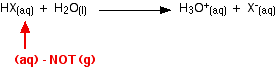 That then needs to be incorporated into an improved energy cycle: 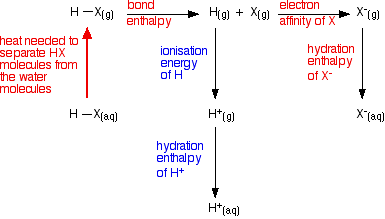 That energy will be much greater in the case of hydrogen fluoride because it forms hydrogen bonds with the water molecules. The other hydrogen halides only form the weaker van der Waals dispersion forces or dipole-dipole attractions. | ||||||||||||||||||||||||||
|
Note: If you aren't sure about van der Waals forces, you could follow this link. Use the BACK button on your browser to return to this page. | ||||||||||||||||||||||||||
Putting all this together, here are the overall enthalpy changes (including all the stages in the energy cycle) for the reactions:
| ||||||||||||||||||||||||||
|
Note: These figures are taken from Modern Physical Chemistry by Liptrot, Thompson and Walker. They are quoted in that book without any explanation of where they came from. I have no way of checking their accuracy. | ||||||||||||||||||||||||||
|
You can see that the enthalpy change for HF is much lower than that
for the other three hydrogen halides - but it is still an exothermic
change. We still haven't got fully to the bottom of why hydrofluoric
acid is a weak acid! Entropy and free energy considerations What decides the extent to which a reaction happens isn't enthalpy change but is a term called free energy change. Free energy change is calculated from the enthalpy change, the temperature of the reaction and the entropy change during the reaction. The only way of making sense of entropy without getting bogged down in some serious maths is to think of it as a measure of the amount of disorder in a system. Entropy is given the symbol S. If a system becomes more disordered, then its entropy increases. If it becomes more ordered, its entropy decreases. The key equation is:  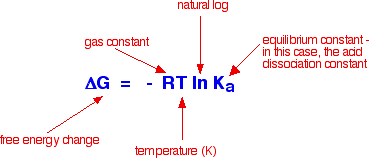 The values for T
| ||||||||||||||||||||||||||
|
Note: These figures are again taken from Modern Physical Chemistry by Liptrot, Thompson and Walker. Again, I don't know how they were calculated, and have no way of checking their accuracy. | ||||||||||||||||||||||||||
|
Notice that at the top of the Group, the systems are becoming more ordered
when the HX reacts with the water. The entropy of the system (the
amount of disorder) is decreasing - particularly with the hydrogen
fluoride. The reason for this is that the very strong attractions between H3O+ and F-(aq) ions imposes a lot of order on the system, as also does the attraction between the water molecules and the various ions present. These attractions (of both kinds) will be at their greatest in the case of the very small fluoride ions. Putting all this together, what is the effect on the free energy change, and therefore the value of the equilibrium constant for the reaction?
By contrast, the estimated equilibrium constant of hydrofluoric acid is pretty small. Hydrofluoric acid only ionises to a very small extent in water. It is a weak acid. It is possible to check the accuracy of our explanation by comparing the real value of the equilibrium constant with the estimated one.
To have the values in close agreement, To be honest, the calculated values for Ka shouldn't really have been quoted beyond 1 significant figure. I've quoted them slightly more accurately than they deserve in order to pick out the trend in the three strong acids. Summary: Why is hydrofluoric acid a weak acid? The two main factors are:
A Peanuts cartoon showed one of the others (Linus?) asking the awful Lucy why the sky was blue. The conversation went something like this:
| ||||||||||||||||||||||||||
Wednesday, 12 June 2013
THE ACIDITY OF THE HYDROGEN HALIDES
Subscribe to:
Post Comments (Atom)
No comments:
Post a Comment